
3-環(huán)己烯-1-甲醛(100-50-5)紅外圖譜(IR1)
InChI:InChI=1S/C7H10O/c8-6-7-4-2-1-3-5-7/h1-2,6-7H,3-5H2
InChIKey: DCFDVJPDXYGCOK-UHFFFAOYSA-N
Smiles:C1(C=O)CCC=CC1
-

Mass
MS-NW-3009
1,2,3,6-tetrahydrobenzaldehyde
C7H10O (Mass of molecular ion: 110)
Source Temperature: 270 °C
Sample Temperature: 180 °C
Reservoir, 75 eV
18.0 1.2
26.0 3.0
27.0 23.8
28.0 2.9
29.0 5.7
31.0 1.5
37.0 1.5
38.0 3.1
39.0 32.3
40.0 4.3
41.0 44.6
42.0 2.4
43.0 2.1
50.0 5.8
51.0 10.9
52.0 5.3
53.0 41.6
54.0 33.7
55.0 12.1
56.0 1.1
57.0 1.5
63.0 1.6
65.0 7.7
66.0 17.0
67.0 32.7
68.0 6.7
69.0 4.6
77.0 23.5
78.0 16.9
79.0 100.0
80.0 13.7
81.0 76.7
82.0 9.9
83.0 2.3
91.0 19.6
92.0 31.0
93.0 4.1
95.0 24.4
96.0 1.6
109.0 7.2
110.0 71.3
111.0 5.8
90 MHz in CDCl3
400 MHz in CDCl3
-

| 1H NMR |
89.56 MHz |
| C7 H10 O
|
0.04 ml : 0.5 ml CDCl3
|
| 1,2,3,6-tetrahydrobenzaldehyde
|
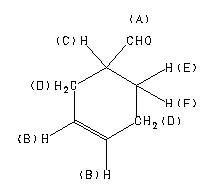 |
Assign. Shift(ppm)
A 9.698
B 5.71
C 2.52
D 2.34 to 2.02
E *1 2.00
F *1 1.67
400MHZ : HSP-41-016
|
Hz ppm Int.
869.06 9.704 821
868.06 9.693 687
512.56 5.724 489
510.94 5.706 1000
510.00 5.695 462
509.44 5.689 525
508.56 5.679 194
231.25 2.583 73
230.06 2.569 81
229.50 2.563 103
228.25 2.549 96
226.50 2.530 90
225.38 2.517 80
223.06 2.491 113
222.69 2.487 99
221.88 2.478 115
221.00 2.468 88
220.00 2.457 71
218.13 2.436 64
217.44 2.428 87
216.19 2.414 94
215.75 2.410 87
215.13 2.403 85
215.00 2.401 85
214.19 2.392 93
213.44 2.384 77
212.63 2.375 62
208.00 2.323 78
206.38 2.305 120
205.25 2.292 185
203.94 2.278 318
202.88 2.266 395
202.25 2.259 351
201.81 2.254 308
201.38 2.249 284
199.69 2.230 203
198.44 2.216 197
197.94 2.211 256
197.44 2.205 249
196.25 2.192 371
195.88 2.188 391
194.63 2.174 259
193.25 2.158 320
192.25 2.147 207
191.69 2.141 258
191.13 2.135 227
190.44 2.127 201
189.50 2.116 247
188.56 2.106 281
187.94 2.099 313
186.88 2.087 440
186.13 2.079 491
185.06 2.067 420
183.94 2.054 326
182.56 2.039 139
181.50 2.027 114
180.75 2.019 105
180.00 2.010 149
179.63 2.006 168
179.00 1.999 171
178.00 1.988 131
176.94 1.976 114
176.56 1.972 142
176.06 1.966 152
175.63 1.962 168
174.63 1.950 132
173.75 1.941 131
173.31 1.936 109
172.56 1.927 140
171.94 1.920 144
170.63 1.906 93
169.50 1.893 101
169.25 1.890 101
167.00 1.865 96
166.44 1.859 85
160.13 1.788 61
158.88 1.775 127
157.44 1.758 63
153.00 1.709 90
151.06 1.687 92
149.75 1.673 100
149.44 1.669 99
149.00 1.664 71
145.94 1.630 64
142.56 1.592 61
-

| 1H NMR |
399.65 MHz |
| C7 H10 O
|
0.04 ml : 0.5 ml CDCl3
|
| 1,2,3,6-tetrahydrobenzaldehyde
|
 |
Assign. Shift(ppm)
A 9.699
B 5.70
C 2.519
D 2.23
E 2.11
F *1 1.999
G *1 1.667
ASSIGNED BY H-H COSY.
J(A,C)=1.2HZ.
|
Hz ppm Int.
3877.11 9.702 689
3876.04 9.699 627
2292.63 5.737 32
2281.80 5.710 542
2280.12 5.706 1000
2278.59 5.702 457
2268.22 5.676 37
2267.30 5.674 44
1021.58 2.557 49
1020.36 2.554 50
1018.22 2.548 54
1017.00 2.545 53
1014.56 2.539 106
1013.34 2.536 109
1011.35 2.531 126
1010.28 2.528 126
1008.30 2.523 69
1007.39 2.521 100
1006.32 2.519 70
1004.49 2.514 149
1003.27 2.511 146
1001.28 2.506 111
1000.06 2.503 103
997.47 2.496 71
996.25 2.493 70
994.26 2.488 68
993.04 2.485 69
899.81 2.252 130
898.44 2.249 136
896.76 2.244 302
895.84 2.242 290
894.32 2.238 223
892.79 2.234 220
889.74 2.227 264
888.52 2.224 265
885.77 2.217 129
852.51 2.134 64
850.37 2.128 114
848.24 2.123 159
846.71 2.119 148
845.03 2.115 201
843.66 2.111 226
842.90 2.110 228
841.67 2.107 196
839.84 2.102 182
838.78 2.099 257
836.64 2.094 159
834.66 2.089 129
833.44 2.086 125
831.76 2.082 67
830.54 2.079 61
811.92 2.032 78
808.56 2.024 79
806.73 2.019 102
803.38 2.011 102
801.54 2.006 48
798.80 1.999 93
795.75 1.992 83
793.76 1.987 125
790.41 1.978 125
788.57 1.974 58
785.06 1.965 54
684.20 1.712 102
677.64 1.696 108
676.42 1.693 113
674.29 1.688 105
671.23 1.680 100
669.71 1.676 101
667.72 1.671 104
666.50 1.668 109
664.67 1.664 93
663.45 1.661 94
661.32 1.655 95
659.79 1.651 93
656.74 1.644 81
654.75 1.639 85
653.53 1.636 89
646.82 1.619 76