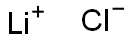?? ??
|
|
?? ?? ??
- ???
- 605 °C (lit.)
- ?? ?
- 1383 °C/1 atm (lit.)
- ?? ??
- 530kg/m3
- ??
- 2.06
- ???
- 1.33 hPa (547 °C)
- ???
- n
20/D 1.381
- ???
- -4 °F
- ?? ??
- 2-8°C
- ???
- H2O: ???
- ?? ?? (pKa)
- 2.256[at 20 ℃]
- ??? ??
- ??
- Specific Gravity
- 2.068
- ??
- ???? ????
- ??????(pH)
- 5.5-7.5 (25℃, 50mg/mL in H2O)
- ??
- ?? ??
- pH ??
- 6
- Flame Color
- Pink/fuchsia
- ???
- 832g/L(20℃)
- ??
- Hygroscopic
- ?? ??(λmax)
- λ: 260 nm Amax: 0.01
λ: 280 nm Amax: 0.01
- Crystal Structure
- NaCl type
- crystal system
- Cube
- Merck
- 14,5528
- Space group
- Fm3m
- Lattice constant
a/nm b/nm c/nm α/o β/o γ/o V/nm3 0.514 0.514 0.514 90 90 90 0.13579
- ???
- ????. ?? ???, ??, ??? ??, ??? ??? ???? ????. ???? ?? ????. ????? ??????.
- InChIKey
- KWGKDLIKAYFUFQ-UHFFFAOYSA-M
- LogP
- -1
- CAS ??????
- 7447-41-8(CAS DataBase Reference)
??
- ?? ? ?? ??
- ?? ? ???? ?? (GHS)
| ??? ?? | Xi | ||
|---|---|---|---|
| ?? ???? ?? | 40-41-62-63-20/21/22 -36/37 -36/37/38 -36/38 | ||
| ????? | 22-26-29-33-36-45-36/37-36/37/39-37/39 | ||
| WGK ?? | 1 | ||
| RTECS ?? | OJ5950000 | ||
| F ?????? | 3-10 | ||
| TSCA | Yes | ||
| HS ?? | 28273980 | ||
| ?? ?? ??? | 7447-41-8(Hazardous Substances Data) | ||
| ?? | LD50 in mice (mg/kg): 990 i.p.; in rats (mg/kg): 600 i.p., 4.8 i.v. (Wielosz). | ||
| ???? ?? | KE-22552 |
?? ?? C??? ??, ??, ??
??
1) ???? ?? ??? ????. 2) ?? ??, ???? ??? ????.??? ??
Lithium chloride is a White cubic crystals; granules or powder; hygroscopic; sharp salt-like taste; melts at 605°C; vaporizes around 1360°C, It has an unusually high water solubility when compared to the other alkali metal chlorides; readily dissolves in water (64g/100mL at 0°C); also highly soluble in alcohol and pyridine; moderately soluble in acetone (4.1 g/100mL at 25°C).The following hydrates are known: LiCl·H2O, LiCl-3H20 and LiCl- 5H2O. The higher hydrates are stable at progressively lower temperatures. Lithium chloride is deliquescent under normal atmospheric conditions.
Lithium chloride is soluble to a significant extent in many polar organic liquids. It is generally most soluble in alcohols in which the solubility decreases as the size of the organic radical increases.

It dehumidifies air for industrial drying and for air conditioning. Lithium chloride bums with a chrims on flame and is used in pyrotechnics. It is also used as a pyrotechnic in welding and brazing fluxes.
??? ??
White cubic crystals; granules or powder; hygroscopic; sharp salt-like taste; refractive index 1.662; density 2.068 g/cm3; melts at 605°C; vaporizes around 1,360°C; readily dissolves in water (64g/100mL at 0°C); also highly soluble in alcohol and pyridine; moderately soluble in acetone (4.1 g/100mL at 25°C).?? ??
Lithium chloride solution can be used:(1) obtaining dendritic cells in the form of LiClPAM3 DCs;
(2) LiCl buffer preparation for immunoprecipitation;
(3) in the preparation of washing buffers;
(4) in the preparation of washing buffers for radioimmunoprecipitation assays (RIPA);
(5) can be used for selective precipitation of RNA.
??
ChEBI: Lithium chloride is a metal chloride salt with a Li(+) counterion. It has a role as an antimanic drug and a geroprotector. It is an inorganic chloride and a lithium salt.?? ??
Lithium chloride may be prepared by reaction of lithium carbonate or lithium hydroxide with hydrochloric acid followed by crystallization:(1) Li2CO3+ 2HCl →2LiCl + CO2+ H2O
(2) LiOH + HCl →LiCl + H2O
Crystallization above 95°C yields anhydrous salt. Hot solution upon cooling forms crystals of monohydrate, LiCl.H2O.
The solid and solution are separated and the supernatant solution is recycled for further evaporation. The crystals are dried to yield anhydrous lithium chloride.
Lithium chloride can be synthesized from its elements by heating lithium metal with chlorine gas.
It also may be obtained from natural brine.
?? ??
Colorless crystals or powder. Low toxicity.??? ?? ??
Very hygroscopic. Very soluble in water.?? ???
These materials have weak oxidizing or reducing powers. Redox reactions can however still occur. For example, CO2, which is often regarded as chemically inert, vigorously oxidizes the strong reducing agent Mg if the two are heated together. The majority of compounds in this class are slightly soluble or insoluble in water. If soluble in water, then the solutions are usually neither strongly acidic nor strongly basic. These compounds are not water-reactive. Some do react with acids: carbonates generate carbon dioxide and heat when treated with acids; fluorides, sulfites and sulfides generate toxic gases (hydrogen fluoride, sulfur dioxide and hydrogen sulfide, respectively) when treated with acids.????
Flash point data for Lithium chloride are not available. Lithium chloride is probably combustible.Safety Profile
Human poison by ingestion. Experimental poison by intravenous and intracerebral routes. Moderately toxic by subcutaneous and intraperitoneal routes. Experimental teratogenic and reproductive effects. Human systemic effects by ingestion: somnolence, tremors, nausea or vomiting. An eye and severe skin irritant. Human mutation data reported. Questionable carcinogen with experimental neoplastigenic data. This material has been recommended and used as a substitute for sodwm chloride in "saltfree" diets, but cases have been reported in which the ingestion of lithium chloride has produced dminess, ringing in the ears, visual disturbances, tremors, and mental confusion. In most cases, the symptoms disappeared when use was discontinued. Prolonged absorption may cause disturbed electrolyte balance, impaired renal function. Reaction is violent with BrF3. When heated to decomposition it emits toxic fumes of Cl-. Used for dehumidification in the air conditioning industry. Also used to obtain lithum metal. See also LITHIUM COMPOUNDS.Purification Methods
Crystallise it from water (1mL/g) or MeOH and dry it for several hours at 130o. Other metal ions can be removed by preliminary crystallisation from hot aqueous 0.01M disodium EDTA. It has also been crystallised from conc HCl, fused in an atmosphere of dry HCl gas, cooled under dry N2 and pulverised in a dry-box. Kolthoff and Bruckenstein [J Am Chem Soc 74 2529 1952] precipitated it with ammonium carbonate, washed it with Li2CO3 five times by decantation and finally with suction, then dissolved it in HCl. The LiCl solution is evaporated slowly with continuous stirring in a large evaporating dish, the dry powder being stored (while still hot) in a desiccator over CaCl2.?? ?? ?? ?? ? ???
???
?? ??
5-CHLORO-2'-DEOXYURIDINE
Methyl 3-cyclopentenecarboxylate
Mometasone furoate
3,5-DIMETHOXYPHENYLACETIC ACID
5-?????-????-2-?
5-Hydroxymethylpyrrolidin-2-one
???? [1,1'- ?? (? ?? ?? ??) ???] ??? (II)
5-????-2-???
(2,5-???-1,3-???-4-?)???
Halobetasol propionate
2-Chloro-6-fluorophenylacetic acid
1-(3-CHLORO-4-((CYCLOPROPYLAMINO)METHYL)PHENYL)ETHANONE
Mn(Ⅲ)salen epoxidation catalyst
???????
2,5-???-1,3-???-4-??????
1,3-???????
1-(3-AMINO-PYRIDIN-2-YL)-ETHANONE
LITHIUM DICHROMATE
optical plastics based on coplymer of diene macromolecule
3-CHLORO-4-((CYCLOPROPYLAMINO)METHYL)BENZALDEHYDE
6-?????
METHYL 2-CHLORO-6-FLUOROPHENYLACETATE
??
?? ?? ?? ??
???( 812)?? ??
| ??? | ?? | ??? | ?? | ?? ? | ?? |
|---|---|---|---|---|---|
| Henan Fengda Chemical Co., Ltd | +86-371-86557731 +86-13613820652 |
info@fdachem.com | China | 20229 | 58 |
| QINGDAO HONG JIN CHEMCIAL CO.,LTD. | 532-83657313 18663969289; |
hjt@hong-jin.com | China | 227 | 58 |
| Xiamen AmoyChem Co., Ltd | +86-86-5926051114 +8615060885618 |
sales@amoychem.com | China | 6383 | 58 |
| Shanghai Worldyang Chemical Co.,Ltd. | +86-021-56795779 +8613651600618 |
sales@worldyachem.com | China | 1210 | 58 |
| Yujiang Chemical (Shandong) Co.,Ltd. | +8617736087130 |
catherine@yjchem.com.cn | China | 994 | 58 |
| Aladdin Scientific | |
tp@aladdinsci.com | United States | 52924 | 58 |
| Shandong Deshang Chemical Co., Ltd. | +86-0531-8875-2665 +8613153039501 |
info@deshangchem.com | China | 662 | 58 |
| Hebei Chuanghai Biotechnology Co., Ltd | +86-15531157085; +8615531157085 |
abby@chuanghaibio.com | China | 8807 | 58 |
| Shaanxi Dideu Medichem Co. Ltd | +86-29-81148696 +86-15536356810 |
1022@dideu.com | China | 3883 | 58 |
| Hebei Chuanghai Biotechnology Co,.LTD | +86-13131129325 |
sales1@chuanghaibio.com | China | 5856 | 58 |