?????? ????? C??? ??, ??, ??
??
Phenazopyridine is an azo dye with analgesic properties. It decreases the bladder distension-induced firing rate of afferent bladder Aγ-fibers, but not C-fibers, in anesthetized rats when administered at a doses of 0.3, 1, and 3 mg/kg. Formulations containing phenazopyridine have been used as analgesics in the treatment of urinary tract infections.
??? ??
Brick-Red Crystals
??
Phenazopyridine hydrochloride has been used for 50 years as an analgesic drug either alone or in combination with other drugs to reduce pain associated with urinary tract infection. An azo dye used in urinary tract infections. Also used as a local anesthetic. Exposure to phenazopyridine hydrochloride occurs during manufacture and formulation.
??
ChEBI: A hydrochloride obtained by combining phenazopyridine with one equivalent of hydrochloric acid. A local anesthetic that has topical analgesic effect on mucosa lining of the urinary tract. Its use is limited by problems with toxicity (primarily blood disord
rs) and potential carcinogenicity.
?? ??
Brick-red microcrystals with a slight violet luster or a purple powder. Aqueous solutions are yellow to brick-red and slightly acidic; they may be stabilized by the addition of 10% glucose. Slightly bitter taste.
??? ?? ??
Dust can be explosive when suspended in air at specific concentrations. Phenazopyridine hydrochloride may be sensitive to air and light. . Very slightly water soluble.
?? ???
Phenazopyridine hydrochloride is an azo compound. Azo, diazo, azido compounds can detonate. This applies in particular to organic azides that have been sensitized by the addition of metal salts or strong acids. Toxic gases are formed by mixing materials of this class with acids, aldehydes, amides, carbamates, cyanides, inorganic fluorides, halogenated organics, isocyanates, ketones, metals, nitrides, peroxides, phenols, epoxides, acyl halides, and strong oxidizing or reducing agents. Flammable gases are formed by mixing materials in this group with alkali metals. Explosive combination can occur with strong oxidizing agents, metal salts, peroxides, and sulfides.
????
Flash point data for Phenazopyridine hydrochloride are not available. Phenazopyridine hydrochloride is probably combustible.
Clinical Use
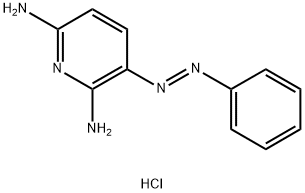
Phenazopyridine hydrochloride, 2,6-diamino-3-(phenylazopyridinehydrochloride (Pyridium), is a brick-red, finecrystalline powder. It is slightly soluble in alcohol, in chloroform,and in water.Phenazopyridine hydrochloride was formerly used asa urinary antiseptic. Although it is active in vitro againststaphylococci, streptococci, gonococci, and E. coli, it has nouseful antibacterial activity in the urine. Thus, its presentutility lies in its local analgesic effect on the mucosa of theurinary tract.
Usually, phenazopyridine is given in combination withurinary antiseptics. For example, it is available as Azo-Gantrisin, a fixed-dose combination with the sulfonamide antibacterialsulfisoxazole, and as Urobiotic, a combination withthe antibiotic oxytetracycline and the sulfonamide sulfamethizole. The drug is rapidly excreted in the urine, towhich it gives an orange-red color. Stains in fabrics may beremoved by soaking in a 0.25% solution of sodium dithionite.
Safety Profile
Confirmed carcinogen with experimental carcinogenic and tumorigenic data. A poison by intraperitoneal and intravenous routes. Moderately toxic by ingestion. Human systemic effects by ingestion: somnolence, cyanosis, dlarrhea, nausea or vomiting, anuria or decreased urine volume, normocytic anemia, methemoglobinemiacarboxyhemoglobinemia, dehydration, changes in blood sodium levels. Mutation data reported. When heated to decomposition it emits very toxic fumes of NOx and HCl
Carcinogenicity
Phenazopyridine hydrochloride is reasonably anticipated to be a human carcinogen based on sufficient evidence of carcinogenicity fromstudies in experimental animals.
?????? ????? ?? ?? ? ???
???
?? ??