Zafirlukast
|
|
Zafirlukast Eigenschaften
- Schmelzpunkt:
- 139°C
- Dichte
- 1.32±0.1 g/cm3(Predicted)
- storage temp.
- room temp
- L?slichkeit
- DMSO: ≥28mg/mL
- pka
- 4.94±0.10(Predicted)
- Aggregatzustand
- solid
- Farbe
- White or pink
- Merck
- 14,10108
- Stabilit?t:
- Stable for 2 years as supplied. Protect from exposure to moisture. Solutions in DMSO may be stored at -20°C for up to 3 months.
- InChIKey
- YEEZWCHGZNKEEK-UHFFFAOYSA-N
- SMILES
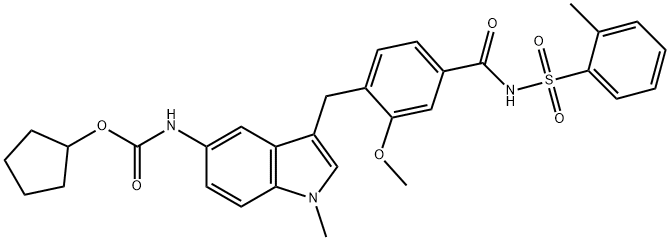
- C(OC1CCCC1)(=O)NC1C=CC2=C(C=1)C(CC1=CC=C(C(NS(C3=CC=CC=C3C)(=O)=O)=O)C=C1OC)=CN2C
- CAS Datenbank
- 107753-78-6(CAS DataBase Reference)
Sicherheit
- Risiko- und Sicherheitserkl?rung
- Gefahreninformationscode (GHS)
| WGK Germany | 3 | ||
|---|---|---|---|
| RTECS-Nr. | FB3966200 | ||
| HS Code | 2935.90.6000 |
| Bildanzeige (GHS) |

|
|||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alarmwort | Warnung | |||||||||||||||||||||||||||||||||||
| Gefahrenhinweise |
|
|||||||||||||||||||||||||||||||||||
| Sicherheit |
|
Zafirlukast Chemische Eigenschaften,Einsatz,Produktion Methoden
Beschreibung
Accolate was launched in Ireland, Finland and the US for treatment of asthma. Prepared via an eight step synthesis from methyl 3-methoxy-4- methylbenzoate, zafirlukast acts as a LTD4 antagonist and is the first compound of a new class of drugs. LTC4, LTD4 and LTE4 were determined to be the constituents of the slow-reacting substance of anaphylaxis (SRS-A) which was found to induce asthma effects (bronchoconstriction, increased vascular permeability resulting in edema, cellular infiltration of airway tissues and decreased mucociliary transport). Thus an inhibitor of their synthesis would at least attenuate these symptoms. Zafirlukast binds to the CysLT receptor LT-1 and blocks the effect of LTC4, LTD4 and LTE4. The drug is an oral twice daily formulation that reversed an LTD4 challenge, attenuated the response of platelet-activating factor (PAF), an allergen and cold air challenge and exercise-induced asthma.Chemische Eigenschaften
Off-white to pale pink crystalline solidVerwenden
Zafirlukast has been used to stimulate pancreatic β cell line (MIN6) and pancreatic islets for insulin secretion assay. It may be used as an adenosine triphosphate-binding cassette transporter (ABCG2) inhibitor in MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] cytotoxicity assay in human embryonic kidney cells (HEK293) and in Kirby-Bauer disc diffusion assays, bactericidal activity and minimal inhibitory concentration (mIC) assay against M. smegmatisAcquired resistance
Zafirlukast inhibits CYP3A4 and CYP2C9 in concentrations equivalent to clinical plasma levels and, therefore, should be used with caution in patients taking drugs metabolized by these enzymes. Specifically, coadministration with warfarin results in a significant increase in prothrombin time. Other drugs metabolized by CYP2C9 are phenytoin and carbamazepine. In addition, CYP3A4-metabolized drugs are cyclosporine, cisapride, and the dihydropyridine class of calcium channel blockers. Of particular interest is the fact that aspirin increases the plasma levels of zafirlukast, and theophylline decreases the plasma levels of zafirlukast. Care should be taken when coadministering with erythromycin, because this decreases the bioavailability of zafirlukast.Clinical Use
Zafirlukast is an indole derivative with a sulfonamide group that fulfills the need for an ionizable moiety on the pharmacophore. A large number of analogues have been prepared; however, they all resulted in a decrease in antagonist activity. Zafirlukast, like montelukast, is a selective antagonist for the cysLT1 receptor and antagonizes the bronchoconstrictive effects of all leukotrienes (LTC4, LTD4, and LTE4).Stoffwechsel
Zafirlukast is well absorbed orally; however, food will decrease its absorption by as much as 40%. Zafirlukast is primarily metabolized in the liver by CYP2C9 and CYP3A4 to hydroxylated metabolites. Zafirlukast also has been shown to undergo carbamate hydrolysis, followed by N-acetylation. Additionally, zafirlukast in known to produce an idiosyncratic hepatotoxicity in susceptible patients. This is appears to result from the formation of an electrophilic α,β-unsaturated iminium intermediate evidenced by the formation of a glutathione adduct on the methylene carbon bridging the indole ring to the methoxybenzene moiety of the molecule. More than 90% if its metabolites are excreted in the feces, with the remaining found in the urine.Zafirlukast Upstream-Materialien And Downstream Produkte
Upstream-Materialien
Natriumhydrid
4-Methylmorpholin
5-Nitroindol
1,4-Dioxan
Tetrahydrofuran
PalladiuM carbon
Naphtha (Erdoel), schwere Alkylat-
Methyljodid
Methyl-m-anisat
Lithiumhydroxid
Disilberoxid
Toluol-2-sulfonamid
Downstream Produkte
Zafirlukast Anbieter Lieferant Produzent Hersteller Vertrieb H?ndler.
Global( 265)Lieferanten
| Firmenname | Telefon | Land | Produktkatalog | Edge Rate | |
|---|---|---|---|---|---|
| BEIJING SJAR TECHNOLOGY DEVELOPMENT CO., LTD. | +86-18600796368 |
sales@sjar-tech.com | China | 485 | 58 |
| Capot Chemical Co.,Ltd. | +8613336195806 |
sales@capot.com | China | 29731 | 60 |
| Henan Tianfu Chemical Co.,Ltd. | +86-0371-55170693 +86-19937530512 |
info@tianfuchem.com | China | 21622 | 55 |
| Hubei Jusheng Technology Co.,Ltd. | 18871490254 |
linda@hubeijusheng.com | CHINA | 28172 | 58 |
| BOC Sciences | +1-631-485-4226 |
inquiry@bocsci.com | United States | 19552 | 58 |
| Alchem Pharmtech,Inc. | 8485655694 |
sales@alchempharmtech.com | United States | 63687 | 58 |
| CONIER CHEM AND PHARMA LIMITED | +8618523575427 |
sales@conier.com | China | 49733 | 58 |
| career henan chemical co | +86-0371-86658258 +8613203830695 |
factory@coreychem.com | China | 29804 | 58 |
| Career Henan Chemica Co | +86-0371-86658258 +8613203830695 |
laboratory@coreychem.com | China | 30230 | 58 |
| HANGZHOU CLAP TECHNOLOGY CO.,LTD | 86-571-88216897,88216896 13588875226 |
sales@hzclap.com | CHINA | 6312 | 58 |
107753-78-6()Verwandte Suche:
Montelukast
3-Methoxybenzylalkohol
Anisol
(Trifluormethoxy)benzol
Phenylvalerat
Sulfanilamid
10,11-Dihydro-α-methyl-10-oxodibenzo[b,f]thiepin-2-essigsure
4-Methoxy-anilin
Phenylsalicylat
Benzolsulfonamid
Diphenylether
Toluol-4-sulfonamid
Methoxyessigs?ure
PHENYL RESIN
Zapizolam
Phenylessigsure
Methoxy
- Carbamic acid, [3-[[2-methoxy-4-[[[(2-methylphenyl)sulfonyl]amino]carbonyl]phenyl]methyl]-1-methyl-1H-indol-5-yl]-, cyclopentyl ester
- Cyclopentyl 3-[2-methoxy-4-[(o-tolylsulfonyl)carbamoyl]benzyl]-1-methylindole-5-carbamate
- [3-[[2-Methoxy-4-[[[(2-methylphenyl)sulfonyl]amino]carbonyl]phenyl]methyl]-1-methyl-1H-indol-5-yl]carbamic acid cyclopentyl ester
- 4-[5-(Cyclopentyloxycarbonylamino)-1-methyl-1H-indol-3-ylmethyl]-3-methoxy-N-(2-methylphenylsulfonyl)benzamide
- IC-204219
- Carbamic acid, N-[3-[[2-methoxy-4-[[[(2-methylphenyl)sulfonyl]amino]carbonyl]phenyl]methyl]-1-methyl-1H-indol-5-yl]-, cyclopentyl ester
- cyclopentyl N-{3-[(2-Methoxy-4-{[(2-Methylbenzene)sulfonyl]carbaMoyl}phenyl)Methyl]-1-Methyl-1H-indol-5-yl}carbaMate
- Cyclopentyl N-[3-{[2-Methoxy-4-[(2-Methylphenyl) sulfonylcarbaMoy
- ZAFIRLUCAST
- Zafirlukast(Accolate)
- Cyclopentyl (3-(2-Methoxy-4-((o-tolylsulfonyl)carbaMoyl)benzyl)-1-Methyl-1H-indol-5-yl)carbaMate
- ICI-2204219
- Vanticon
- [3-[[2-Methoxy-4-[[[(2-Methylphenyl-d7)sulfonyl]aMino]carbonyl]phenyl]Methyl]-1-Methyl-1H-indol-5-yl]carbaMic Acid Cyclopentyl Ester
- Zafilukast
- Accolate
- ICI-204219
- N-[3-[[2-methoxy-4-[[[(2-methylphenyl)sulfonyl]amino]carbonyl]phenyl]methyl]-1-methyl-1H-indol-5-yl]-carbamic acid cyclopentyl ester
- Zafirlukast, >=99%
- Cyclopentyl (3-(2-methoxy-4-((o-tolylsulfonyl)-carbamoyl)benzyl)-1-methyl-1H-indol-5-yl)carbam
- ZAFIRLUKAST
- [3-[[2-Methoxy-4-[[[(2-methylphenyl)sulfonyl]amino]carbonyl]phenyl]methyl]-1-methyl-1H-indol-5-y1]carbamic acid eyclopentyl ester
- cyclopentyl n-[3-{[2-methoxy-4-[(2-methylphenyl) sulfonylcarbamoyl]phenyl]methyl}-1-methyl-indol-5-yl]carbamate
- cyclopentyl [3-[[2-methoxy-4-[(2-methylphenyl)sulfonylcarbamoyl]phenyl]methyl]-1-methyl-indol-5-yl]aminoformate
- Zafirlukast Tablet
- [3-[[2-Methoxy-4-[[[(2-methylphenyl)sulfonyl]amino]carbonyl]phenyl]methyl]-1-methyl-1H-indol-5-yl]carbamic Acid Cyclopentyl Ester, ICI-2204219, Accolate
- Zafirlukast (ICI 204219)
- CS-79
- zafirlukas
- Cyclopentyl N-[3-[[2-Methoxy-4-[[[(2-methylphenyl)sulfonyl]amino]carbonyl]phenyl]methyl]-1-methyl-1H-indol-5-yl]carbamate
- Zafirlukast USP/EP/BP
- ZafirlukastQ: What is Zafirlukast Q: What is the CAS Number of Zafirlukast Q: What is the storage condition of Zafirlukast Q: What are the applications of Zafirlukast
- Zafirlukast 13CD3Q: What is Zafirlukast 13CD3 Q: What is the CAS Number of Zafirlukast 13CD3 Q: What is the storage condition of Zafirlukast 13CD3 Q: What are the applications of Zafirlukast 13CD3
- ZafirlukastStandard IHS
- Zafirlukast, 10 mM in DMSO
- Zafirlukast - Bio-X ?
- 107753-78-6
- C31H33N3O6S
- Zafirlukast
- All Inhibitors
- Inhibitors
- Intermediates & Fine Chemicals
- Pharmaceuticals
- APIs
- Heterocycles
- Sulfur & Selenium Compounds
- Accolate, Accoleit, Vanticon