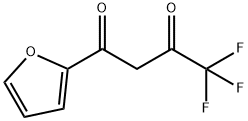
| Identification | More | [Name]
4,4,4-TRIFLUORO-1-(2-FURYL)-1,3-BUTANEDIONE | [CAS]
326-90-9 | [Synonyms]
1,3-Butanedione, 4,4,4-trifluoro-1-(2-furanyl)-
1,3-Butanedione, 4,4,4-trifluoro-1-(2-furyl)-
4,4,4-trifluoro-1-(2-furanyl)-3-butanedione
Furonyltrifluoroacetone
Furoyltrifluoroacetone
Perfluoroacetyl(2-furoyl)methane
Furoyltrifluoroacetone~1-(2-Furyl)-4,4,4-trifluoro-1,3-butanedione
4,4,4-Trifluoro-1-(2-furyl)butane-1,3-dione 99%
4,4,4-Trifluoro-1-(2-furyl)butane-1,3-dione99%
4,4,4-Trifluoromethyl-1-(2-furyl)-1,3-butandione, GC 99%
4,4,4-TRIFLUORO-1-(2-FURYL)-1,3-BUTANEDIONE 99%
1-(2-Furanyl)-4,4,4-trifluoro-1,3-butanedione
1-(2-Furyl)-4,4,4-trifluoro-1,3-butanedione
2-Furoyltrifluoroacetonee
4,4,4-Trifluoro-1-(2-furanyl)-1,3-butanedione | [EINECS(EC#)]
206-315-1 | [Molecular Formula]
C8H5F3O3 | [MDL Number]
MFCD00020935 | [Molecular Weight]
206.119 | [MOL File]
326-90-9.mol |
| Chemical Properties | Back Directory | [Appearance]
clear yellow liquid | [Melting point ]
19-21 °C(lit.) | [Boiling point ]
203 °C(lit.) | [density ]
1.391 g/mL at 25 °C(lit.)
| [refractive index ]
n20/D 1.528(lit.)
| [Fp ]
189 °F
| [storage temp. ]
Refrigerator (+4°C) | [form ]
powder to lump to clear liquid | [pka]
5.56±0.23(Predicted) | [color ]
White or Colorless to Yellow to Orange | [FreezingPoint ]
20.0 to 22.0 ℃ | [BRN ]
168085 | [InChI]
InChI=1S/C8H5F3O3/c9-8(10,11)7(13)4-5(12)6-2-1-3-14-6/h1-3H,4H2 | [InChIKey]
OWLPCALGCHDBCN-UHFFFAOYSA-N | [SMILES]
C(C1=CC=CO1)(=O)CC(=O)C(F)(F)F | [CAS DataBase Reference]
326-90-9(CAS DataBase Reference) | [EPA Substance Registry System]
1,3-Butanedione, 4,4,4-trifluoro-1-(2-furanyl)- (326-90-9) |
| Safety Data | Back Directory | [Hazard Codes ]
Xi | [Risk Statements ]
R36/37/38:Irritating to eyes, respiratory system and skin . | [Safety Statements ]
S26:In case of contact with eyes, rinse immediately with plenty of water and seek medical advice .
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection . | [WGK Germany ]
3
| [Hazard Note ]
Irritant | [TSCA ]
Yes | [HazardClass ]
IRRITANT | [HS Code ]
29321900 |
| Hazard Information | Back Directory | [Chemical Properties]
clear yellow liquid | [Uses]
4,4,4-Trifluoro-1-(2-furyl)-1,3-butanedione (tfa) may be used in the following studies:
- As capping ligand in the synthesis of [Eu(tfa)3]2bpm complexes (bpm=2,2′-bipyrimidine).
- As reagent in the multistep synthesis of [13CD2]benzylamine.
- As reagent in the synthesis of 3-trifluoromethyl-2-arylcarbonylquinoxaline 1,4-di-N-oxide derivatives by reacting with corresponding benzofurazan oxides.
- In the efficient syntheses of perfluoroalkyl substituted azoles.
- Synthesis of 2-arylcarbonyl-3-trifluoromethylquinoxaline 1,4-di-N-oxide derivatives.
| [General Description]
4,4,4-Trifluoro-1-(2-furyl)-1,3-butanedione (furoyltrifluoroacetone, FTFA) is a β-diketone. Its cytotoxic activity against human cultured tumor and normal cells has been evaluated. Reports suggest that 4,4,4-trifluoro-1-(2-furyl)-1,3-butanedione partially inhibits the oxidation of ferrocyanide in ETP (electron transport particles) isolated from beef heart mitochondria. Its reaction with N,N,N′,N′-tetramethylalkyl diamines to form ionic adducts has been investigated. The conformational analysis of the enol and keto form of FTFA has been reported. | [Synthesis]
GENERAL STEPS: To a 500 mL three-necked flask were added 20.0 g (181.6 mmol) of 2-acetylfuran, 50 mL of tetrahydrofuran (THF) and 24 mL of ethyl trifluoroacetate. The mixed solution was cooled to 0-3 °C in an ice-water bath, and 1.0 M lithium bis(trimethylsilyl)amide (LiHMDS, 200 mL) solution was slowly added dropwise. After the dropwise addition, the reaction system was allowed to warm up to room temperature naturally and stirred overnight. Upon completion of the reaction, THF was removed by concentration under reduced pressure with a rotary evaporator. the residue was transferred to a partition funnel and extracted with a solution of ethyl acetate and 1.0 M hydrochloric acid. The organic phase was separated, washed with saturated saline, dried over anhydrous sodium sulfate and concentrated again under reduced pressure. The final product was 4,4,4-trifluoro-1-(2-furyl)-1,3-butanedione as a brown semi-solid in a yield of 32.5 g (yield >100%). | [References]
[1] Patent: WO2008/73825, 2008, A1. Location in patent: Page/Page column 134
[2] Chinese Chemical Letters, 2016, vol. 27, # 4, p. 566 - 570
[3] Synthesis, 1997, # 11, p. 1321 - 1324
[4] Journal of the American Chemical Society, 1950, vol. 72, p. 2948,2949
[5] Journal of Fluorine Chemistry, 2002, vol. 118, # 1-2, p. 135 - 147 |
| Spectrum Detail | Back Directory | [Spectrum Detail]
4,4,4-TRIFLUORO-1-(2-FURYL)-1,3-BUTANEDIONE(326-90-9)MS
4,4,4-TRIFLUORO-1-(2-FURYL)-1,3-BUTANEDIONE(326-90-9)1HNMR
4,4,4-TRIFLUORO-1-(2-FURYL)-1,3-BUTANEDIONE(326-90-9)13CNMR
4,4,4-TRIFLUORO-1-(2-FURYL)-1,3-BUTANEDIONE(326-90-9)IR1
4,4,4-TRIFLUORO-1-(2-FURYL)-1,3-BUTANEDIONE(326-90-9)IR2
|
|
|