| Identification | More | [Name]
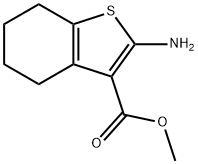
2-AMINO-4,5,6,7-TETRAHYDRO-BENZO[B]THIOPHENE-3-CARBOXYLIC ACID METHYL ESTER | [CAS]
108354-78-5 | [Synonyms]
1-BENZOTHIOPHENE-3-CARBOXYLIC ACID, 2-AMINO-4,5,6,7-TETRAHYDRO-, METHYL ESTER
2-AMINO-4,5,6,7-TETRAHYDRO-BENZO[B]THIOPHENE-3-CARBOXYLIC ACID METHYL ESTER
AKOS B000467
AKOS BBB/011
AKOS BBS-00000703
ART-CHEM-BB B000467
ASISCHEM Z95321
CHEMBRDG-BB 3000467
METHYL 2-AMINO-4,5,6,7-TETRAHYDRO-1-BENZOTHIOPHENE-3-CARBOXYLATE | [Molecular Formula]
C10H13NO2S | [MDL Number]
MFCD00130099 | [Molecular Weight]
211.28 | [MOL File]
108354-78-5.mol |
| Hazard Information | Back Directory | [Synthesis]
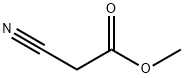
Methyl 2-amino-4,5,6,7-tetrahydrobenzo[b]thiophene-3-carboxylate (Compound 1) was synthesized from cyclohexanone and methyl cyanoacetate by standard Gewald reaction. The procedure was as follows: to a stirred solution of cyclohexanone (4.91 g, 0.05 mol), methyl cyanoacetate (4.95 g, 0.05 mol), and sulfur (1.92 g, 0.06 mol) in 35 mL of ethanol, morpholine (4.4 g, 0.05 mol) was slowly added dropwise. After the dropwise addition was completed, the reaction mixture was refluxed for 3 hours. After completion of the reaction, it was cooled to room temperature and the precipitate was collected by filtration. Purification by ethanol recrystallization gave a light yellow powdery product (8.6 g, 82% yield). The melting point of the product was 128.2-129.4 °C (literature value: 128-130 °C), and mass spectrometry (GC-MS) analysis showed a m/z of 211 [M + H]+ and fragment ion peaks of 179, 151, 125, 91, 77, 65, 53. | [References]
[1] Asian Journal of Chemistry, 2010, vol. 22, # 9, p. 7399 - 7404
[2] Organic and Biomolecular Chemistry, 2014, vol. 12, # 12, p. 1942 - 1956
[3] Bioorganic and Medicinal Chemistry, 2016, vol. 24, # 8, p. 1866 - 1871
[4] Tetrahedron, 2006, vol. 62, # 29, p. 7121 - 7131
[5] Bioorganic and Medicinal Chemistry Letters, 2016, vol. 26, # 8, p. 1947 - 1953 |
|
|