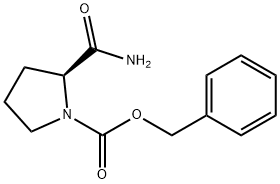
Z-PRO-NH2 synthesis
- Product Name:Z-PRO-NH2
- CAS Number:34079-31-7
- Molecular formula:C13H16N2O3
- Molecular Weight:248.28

1148-11-4

34079-31-7
General procedure for the synthesis of (S)-1-N-benzyloxycarbonylproline from N-benzyloxycarbonyl-L-proline: 1-{[(phenylmethyl)oxy]carbonyl}-L-proline (8.0 g, 32.09 mmol) was solubilized in 1,4-dioxane (40 mL), and pyridine (1.5 mL) and di-tert-butyl dicarbonate ((Boc)2O, 9.1 g 41.72 mmol). Ammonium bicarbonate (3.2 g, 40.43 mmol) was then added. The reaction mixture was stirred at room temperature overnight and then diluted with ethyl acetate (100 mL) and washed sequentially with water (50 mL) and 5% sulfuric acid (H2SO4). The organic layer was separated, dried over anhydrous sodium sulfate (Na2SO4) and filtered. The solvent was removed by distillation under reduced pressure to give a clarified oil. The oily material was ground with ether, the precipitate was collected by filtration and dried under vacuum to afford (S)-1-N-Benzyloxycarbonylprolinamide as a white solid (3.9 g, 49% yield). The mass spectrum showed [M+H]+ of 249.
501-53-1
416 suppliers
$10.00/1g

34079-31-7
197 suppliers
$19.00/5 g
Yield:-
Steps:
Multi-step reaction with 2 steps
1: sodium hydroxide / H2O / Ambient temperature
2: 1.) triethylamine, isobutyl chloroformate, 2.) ammonia / 1.) chloroform, 0 deg C, 2 h, 2.) chloroform, RT, overnight
References:
Morikawa;Honda;Endoh -i.;Matsumoto;Akamatsu -i.;Mitsui;Koizumi [Journal of Pharmaceutical Sciences,1991,vol. 80,# 9,p. 837 - 842]

1148-11-4
464 suppliers
$6.00/10g

34079-31-7
197 suppliers
$19.00/5 g

501-53-1
416 suppliers
$10.00/1g

7531-52-4
686 suppliers
$6.00/5g

34079-31-7
197 suppliers
$19.00/5 g
3304-59-4
80 suppliers
$11.65/5G

34079-31-7
197 suppliers
$19.00/5 g

147-85-3
1064 suppliers
$6.00/25g

34079-31-7
197 suppliers
$19.00/5 g