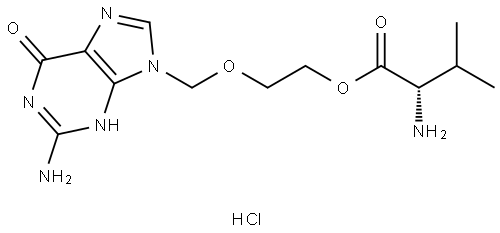
Valacyclovir hydrochloride synthesis
- Product Name:Valacyclovir hydrochloride
- CAS Number:124832-27-5
- Molecular formula:C13H20N6O4.ClH
- Molecular Weight:360.8
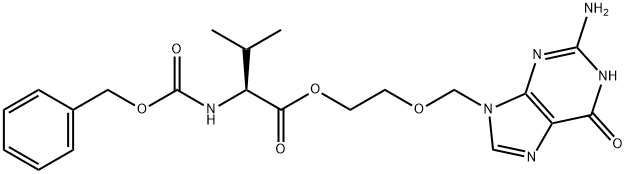
124832-31-1

124832-27-5
General procedure: To a 1L hydrogenation kettle was added (S)-2-((2-amino-6-oxo-1H-purin-9(6H)-yl)methoxy)ethyl 2-(((benzyloxy)carbonyl)amino)-3-methylbutanoate (50g), concentrated hydrochloric acid (9.6mL), 5% palladium-carbon catalyst (5g) and water (350mL), and the reaction was carried out for 6 to 12 hours under the conditions of hydrogen pressure of 3kg/cm2 and temperature of 25-30°C. -30°C for 6 to 12 hours. Upon completion of the reaction, the reaction mixture was vacuum filtered and the filter cake was washed with water. The filtrate was concentrated under reduced pressure and subsequently cooled to room temperature. Isopropanol was added to the concentrate, which was further cooled to 5-10 °C and maintained at that temperature for 1 hour. The precipitated solid was (S)-2-((2-amino-6-oxo-1H-purin-9(6H)-yl)methoxy)ethyl 2-amino-3-methylbutyrate hydrochloride, which was filtered and washed with cold isopropanol to give the product. The yield was 92% and the purity was 99.8%. Analytical conditions: Chromatographic column: USP L1, 4.6×150 mm, 3.5 μm (Zorbax SB-C18, item 863953-914) Detector: UV 250nm Mobile phase A: In a 1000mL volumetric flask, add 2.8mL triethylamine with 800mL ultrapure water, adjust pH to 5.00±0.05 with glacial acetic acid, and settle to scale. Mobile phase B: acetonitrile Injection volume: 50μL Column temperature: 30°C Flow rate: 1.0mL/min Gradient elution

124832-31-1
118 suppliers
$149.00/50mg

124832-27-5
507 suppliers
$7.00/10mg
Yield:124832-27-5 92%
Reaction Conditions:
with hydrogenchloride;5%-palladium/activated carbon;hydrogen in water at 25 - 30; under 2250.23 - 6000.6 Torr;Concentration;
Steps:
2
To a 1L capacity hydrogenator charged a compound of formula II (50g), concentrated hydrochloric acid (9.6ml), 5% palladium on carbon (5g) and water (350ml) and hydrogenate the reaction mass under hydrogen pressure of 3 kg/cm 2 to 8 kg/cm 2 at a temperature of 25-30°C for 6 to 12 hours. The reaction mass was filtered under vacuum and washed with water. The filtrate was then distilled off under vacuum. The reaction mass was then cooled and isopropyl alcohol was added to the reaction mass. The reaction mass was further cooled to a temperature of 5-10°C and maintained for 1 hour. The product valacyclovir hydrochloride obtained was filtered and washed with isopropyl alcohol.Yield 92% and purity 99.8%ANALYTICAL METHOD FOR ANALYSISHPLC column : USP LI, 4.6 x 150 mm, 3.5 ???(Zorbax SB-C18, Part No. 863953-914)Detector : UV 250nmMobile phase A : In a 1000ml volumetric flask add 2.8ml of triethylamine, 800ml of purified water and adjust the pH to 5.00 + 0.05 with glacial acetic acid. Complete the volume with purified water.Mobile Phase B AcetonitrileInjection volume 50??,Column temperature 30°CFlow rate l.OmL/minutesGradient
References:
WO2013/76688,2013,A1 Location in patent:Page/Page column 10-11

502421-44-5
32 suppliers
$40.00/50mg

124832-27-5
507 suppliers
$7.00/10mg
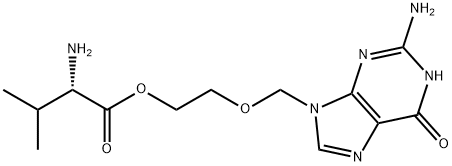
124832-26-4
219 suppliers
$49.00/50mg

124832-27-5
507 suppliers
$7.00/10mg
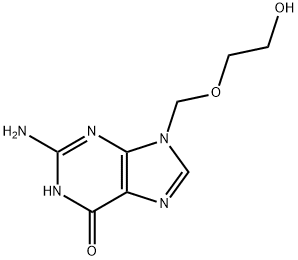
59277-89-3
892 suppliers
$5.00/250mg

124832-27-5
507 suppliers
$7.00/10mg
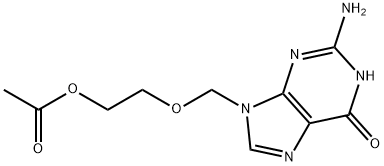
102728-64-3
115 suppliers
$65.00/25mg

124832-27-5
507 suppliers
$7.00/10mg