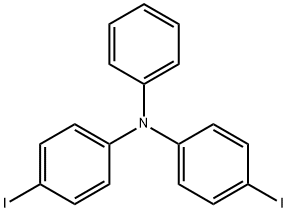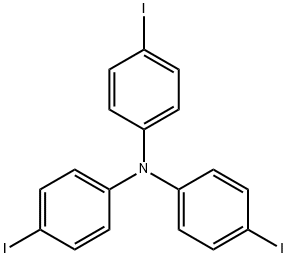
Tris(4-iodophenyl)amine synthesis
- Product Name:Tris(4-iodophenyl)amine
- CAS Number:4181-20-8
- Molecular formula:C18H12I3N
- Molecular Weight:623.01
603-34-9

4181-20-8
The general procedure for the synthesis of tris(4-iodophenyl)amine was as follows: first, IPe2BF4 (5.3 g, 14.3 mmol) and triphenylamine (1 g, 4.1 mmol) were mixed in CH2Cl2 (60 mL) and cooled to 0 °C under nitrogen protection. Subsequently, trifluoromethanesulfonic acid (TfOH: 900 μL, 4.1 mmol) was added slowly and dropwise. The reaction mixture was stirred at room temperature under nitrogen atmosphere for 21 h until a reddish brown solution was formed. After completion of the reaction, saturated aqueous NaHCO3 and Na2S2O3 were added to the mixture and the aqueous layer was extracted with CH2Cl2. The organic layers were combined and washed sequentially with saturated NaHCO3 and NaCl solutions and then dried with Na2SO4. The dried organic phase was filtered and concentrated to give the crude product. The crude product was purified by silica gel column chromatography (eluent: hexane/EtOAc = 5/1) to afford tris(4-iodophenyl)amine (2.507 g, 99% yield). The product was characterized by 1H NMR and 13C NMR: 1H NMR (CDCl3) δ 7.54 (d, J = 8.9 Hz, 6H), 6.81 (d, J = 8.9 Hz, 6H); 13C NMR (CDCl3) δ 146.5, 138.4, 126.0. The NMR data confirm that the resulting compound is tris(4-iodophenyl)amine.

603-34-9
314 suppliers
$24.89/5g

4181-20-8
202 suppliers
$9.00/1g
Yield:4181-20-8 99%
Reaction Conditions:
Stage #1:N,N-diphenylaminobenzene with trifluorormethanesulfonic acid;[bis(pyridine)iodine]+ tetrafluoroborate in dichloromethane at 0 - 20; for 21 h;
Stage #2: with water;sodium thiosulfate in dichloromethane
Steps:
4
Synthesis Example 4Tris(4-iodophenyl)amine was prepared as follows. Firstly, a mixture of IPy2BF4 (5.3 g, 14.3 mmol) and triphenylamine (1 g, 4.1 mmol) was added with dist. CH2Cl2 (60 mL) under a nitrogen atmosphere, and then was added dropwise at 0° C. with trifluoromethanesulfonic acid (TfOH: 900 μL, 4.1 mmol). Thereafter, the resultant mixture was stirred under a nitrogen atmosphere at room temperature for 21 hours to obtain a reddish-brown reaction mixture. Subsequently, the obtained reaction mixture was added with sat. Na2S2O3, and an aqueous layer wad extracted with CH2Cl2. After that, an organic layer thus collected was washed with sat. NaCl, and dried with Na2SO4. Subsequently, the dried organic layer was filtered and concentrated to obtain a crude product. Then, the obtained crude product was separated and purified by silica gel column chromatography (hexane/EtOAc=5/1) to obtain tris(4-iodophenyl) amine (2.507 g, 99% yield).The obtained compound was subjected to 1H NMR and 13C NMR measurements. The obtained results are shown below.1H NMR (CDCl3) δ7.54 (d, J=8.9 Hz, 6H), 6.81 (d, J=8.9 Hz, 6H).13C NMR (CDCl3) δ146.5, 138.4, 126.0, 86.6.From the NMR measurement results, it was confirmed that the obtained compound was tris(4-iodophenyl)amine.
References:
KABUSHIKI KAISHA TOYOTA CHUO KENKYUSHO;TOYOSHI SHIMADA US2008/227939, 2008, A1 Location in patent:Page/Page column 17

122-39-4
358 suppliers
$14.00/5g

4181-20-8
202 suppliers
$9.00/1g
108-86-1
525 suppliers
$10.00/5g

4181-20-8
202 suppliers
$9.00/1g