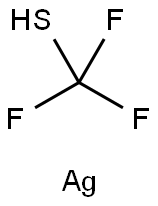
Silver(I) trifluoromethanethiolate synthesis
- Product Name:Silver(I) trifluoromethanethiolate
- CAS Number:811-68-7
- Molecular formula:CHAgF3S
- Molecular Weight:209.94

14104-20-2
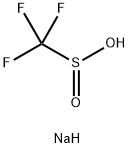
2926-29-6

811-68-7
Silver tetrafluoroborate (AgBF4, 19.77 g, 100 mmol) and sodium trifluoromethanesulfinate (CF3SO2Na, 18.73 g, 120 mmol) were dissolved in anhydrous acetonitrile (400 mL) under nitrogen protection. After cooling the reaction mixture to 0°C, triphenylphosphine (Ph3P, 62.95 g, 240 mmol) was slowly added. Subsequently, the reaction mixture was brought back to room temperature and heated to 50°C and maintained at this temperature for 12 hours. Upon completion of the reaction, the mixture was filtered through diatomaceous earth and the filtrate was cooled to -20°C to promote crystallization. After the crystals were sufficiently precipitated, the supernatant was decanted to remove most of the triphenylphosphine oxide. The supernatant was concentrated under reduced pressure to remove the solvent and the residue was washed well with ether and dried under vacuum. Finally, the resulting solid was recrystallized with tetrahydrofuran and benzene to afford silver trifluoromethane thiol (I) (18.60 g, 89% yield).
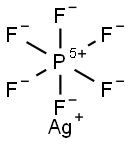
26042-63-7
121 suppliers
$25.00/1 g

2926-29-6
310 suppliers
$6.00/5g

811-68-7
73 suppliers
$27.00/100mg
Yield:811-68-7 86%
Reaction Conditions:
in acetonitrile at 0 - 20; for 12 h;Inert atmosphere;
Steps:
11
Under a nitrogen atmosphere, AgPF6 (25.28 g,100 mmol ) and CF3SO2Na (18.73 g,120 mmol) dissolved in anhydrous acetonitrile (400mL), the reaction solution was cooled to 0°C, followed by the addition of Ph3P (62.95 g, 240 mmol). The reaction solution was returned to room temperature and stirred at room temperature for 12 hours. After the reaction, the reaction solution was filtered through celite, the filtrate was cooled to -20 ° C, and crystals were precipitated. The supernatant was purged to remove most of the triphenylphosphine oxide. The supernatant was removed in vacuo to remove the solvent. The residue was washed thoroughly with ether, vacuum-dried and the residual solid was recrystallized from acetonitrile and toluene to give the product trifluoromethylthio silver (I)(17.97 g, yield 86%).
References:
Zhao, Fangfei;Yang, Yi CN105017110, 2016, B Location in patent:Paragraph 0041; 0042

14104-20-2
193 suppliers
$19.00/1g

2926-29-6
310 suppliers
$6.00/5g

811-68-7
73 suppliers
$27.00/100mg
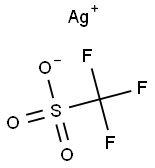
2923-28-6
361 suppliers
$6.00/1g

2926-29-6
310 suppliers
$6.00/5g

811-68-7
73 suppliers
$27.00/100mg

75-15-0
267 suppliers
$40.00/100 g

811-68-7
73 suppliers
$27.00/100mg

