
THYMINE-1-ACETIC ACID synthesis
- Product Name:THYMINE-1-ACETIC ACID
- CAS Number:20924-05-4
- Molecular formula:C7H8N2O4
- Molecular Weight:184.15
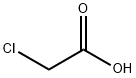
79-11-8
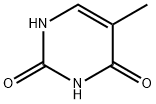
65-71-4

20924-05-4
5-Methyluracil (10.0 g, 79.3 mmol) was suspended in distilled water (150 mL). Aqueous potassium hydroxide solution (50 mL, 3.6 M) was slowly added to the suspension. The mixture was stirred at room temperature for 10 minutes until the solution gradually became clear. Subsequently, chloroacetic acid (15.0 g, 159 mmol) was added to the clarified solution. The reaction mixture was heated to reflux and kept for 90 minutes. Upon completion of the reaction, the solution was cooled to room temperature and acidified with concentrated hydrochloric acid to pH=3. The acidified solution was placed in a refrigerator at 4°C overnight to promote the formation of white crystalline precipitate. The white crystalline precipitate was collected by filtration and dried under vacuum using phosphorus pentoxide (P2O5), resulting in 4.5 g of thymine-1-acetic acid in 31% yield.
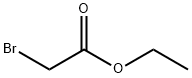
105-36-2
358 suppliers
$5.00/5 g

65-71-4
536 suppliers
$5.00/5g

20924-05-4
129 suppliers
$9.00/250mg
Yield: 327 grams (75%)
Reaction Conditions:
with hydrogenchloride;sodium hydroxide;ammonium sulfate;1,1,1,3,3,3-hexamethyl-disilazane in methanol;acetonitrile
Steps:
5 Synthesis of 2-(1'-thyminyl)acetic acid
Example 5 Synthesis of 2-(1'-thyminyl)acetic acid To 300 grams of thymine was added 750 mL hexamethyldisilazane and 15 grams of ammonium sulfate. The stirring reaction was heated at reflux until no more gas was evolved. The reaction was then cooled to 80° C. and then 160 mL of ethylbromoacetate was added. Gentle heating of the reaction was continued until tlc analysis indicated that 95% of the starting material was consumed. The reaction was then cooled to 20° C. in an ice bath and then 150 mL of methanol was added dropwise while stirring in the ice bath. Once the addition of methanol was complete, 2.5 liters of 3N NaOH was added carefully. The ice bath was then removed and gas was passed over the reaction rapidly to vaporize excess silanes. Then 80 grams of sodium hydroxide was added and the reaction was allowed to stir overnight. In the morning, 1 L of 6N HCl was added to the reaction to cause the product to crystallize. The product was then collected by vacuum filtration and washed with dilute aqueous hydrochloric acid. The collected product was then suspended in 1.5 L of acetonitrile and the solution allowed to reflux with stirring. After cooling overnight, the solid was again collected by vacuum filtration, washed with acetonitrile, dichloromethane, and acetonitrile. Yield: 327 grams (75%). 1 H--NMR (d6 DMSO): δ=12.06 (s, 1H), 11,31 (s, 1H), 7.48 (s,1H), 4.35 (s, 2H), 1.74 (s, 3H)
References:
PerSeptive Biosystems, Inc. US6063569, 2000, A

55036-34-5
3 suppliers
inquiry

20924-05-4
129 suppliers
$9.00/250mg
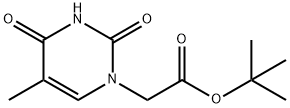
272788-85-9
2 suppliers
inquiry

20924-05-4
129 suppliers
$9.00/250mg
