Tetracaine synthesis
- Product Name:Tetracaine
- CAS Number:94-24-6
- Molecular formula:C15H24N2O2
- Molecular Weight:264.36

109-65-9
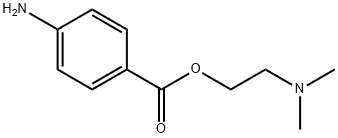
10012-47-2

94-24-6
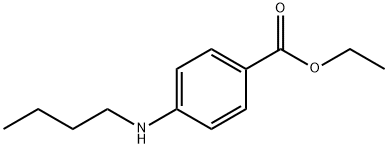
General procedure for the synthesis of 2-(dimethylamino)ethyl 4-(butylamino)benzoate from bromobutane and 2-(dimethylamino)ethyl 4-aminobenzoate: 5.0 g of p-aminobenzoic acid-2-dimethylaminoethyl ester (0.024 mol), 3.3 g of 1-bromonobutane (0.024 mol), 10.0 g of K2CO3 (0.072 mol) and 50 mL of N,N-dimethylformamide were added to a 100 mL three-neck flask. ) and 50 mL of N,N-dimethylformamide were added to a 100 mL three-neck flask. The mixture was stirred and heated to 50 °C, keeping the reaction temperature at 50 °C. After 5 hours of reaction, the heating was stopped and the reaction system was allowed to cool to room temperature. The reaction mixture was filtered and the filtrate was extracted with 50 mL of dichloromethane. The aqueous phase was extracted again with 50 mL of dichloromethane. The organic phases were combined and 5.3 g of white solid product was obtained in 82.8% yield after evaporating the solvent.
Yield:94-24-6 82.8%
Reaction Conditions:
with potassium carbonate in N,N-dimethyl-formamide at 50; for 5 h;
Steps:
3 Preparation of tetracaine (7)
5.0 g of p-aminobenzoic acid-2-dimethylaminoethyl ester (5) (0.024 mol 1)3.3g1-Bromo-n-Butane(0 · 024mo 1), 10.0g K2C03 (0 · 072mo 1) 50mL N, N-dimethylformamide was placed in 1OOmL three-necked flask, stirred and heated to 50 ° C, the reaction system was maintained at 50 After the reaction was stopped for 5 h, the reaction was stopped and the reaction system was allowed to stand at room temperature. The filtrate was extracted with 50 mL of dichloromethane. The aqueous phase was extracted with 50 mL of dichloromethane. The organic phase was combined, To give 5.3 g of a white solid, yield 82.8%.
References:
CN106518697,2017,A Location in patent:Paragraph 0014
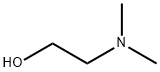
108-01-0
0 suppliers
$10.00/20mg
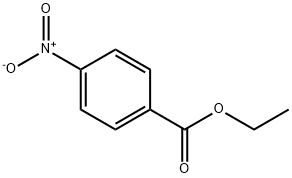
99-77-4
218 suppliers
$12.00/1mg

123-72-8
438 suppliers
$12.32/25ML

94-24-6
413 suppliers
$25.00/1mg

108-01-0
0 suppliers
$10.00/20mg

4740-24-3
120 suppliers
$18.00/250mg

94-24-6
413 suppliers
$25.00/1mg

94-09-7
0 suppliers
$5.00/50mg

94-24-6
413 suppliers
$25.00/1mg