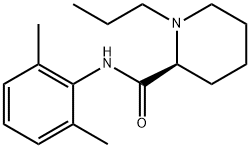
Ropivacaine synthesis
- Product Name:Ropivacaine
- CAS Number:84057-95-4
- Molecular formula:C17H26N2O
- Molecular Weight:274.4
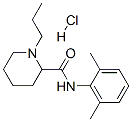
98717-15-8

84057-95-4
The general procedure for the synthesis of (S)-N-(2,6-dimethylphenyl)-1-propylpiperidine-2-carboxamide hydrochloride as raw material for the synthesis of (S)-N-(2,6-dimethylphenyl)-1-propyl-2-piperidine carboxamides was as follows: firstly, 1 g of (S)-N-(2,6-dimethylphenyl)-1-propylpiperidine-2-carboxamide hydrochloride was dissolved in 35 g of USP-grade purified water . Subsequently, the pH was adjusted to 9-10 by slow dropwise addition of about 4 mL of 1.0 N sodium hydroxide solution.The precipitate precipitated from the reaction mixture was collected by filtration and washed with purified water. The resulting solid was re-dispersed in 50 mL of purified water, stirred for 15 min and filtered again and washed with purified water. After repeating the above dispersion, filtration and washing operations, the product was dried at 40 °C for 72 h to obtain the target compound (S)-N-(2,6-dimethylphenyl)-1-propyl-2-piperidine carboxamide.

106-94-5
587 suppliers
$10.00/5ml
27262-40-4
312 suppliers
$11.00/5g

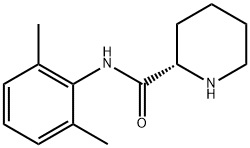
84057-95-4
221 suppliers
$49.00/10mg
Yield:84057-95-4 94%
Reaction Conditions:
in tetrahydrofuran; for 20 - 24 h;Heating / reflux;
Steps:
1
Example 1. Preparation of Ropivacaine baseRopivacaine is prepared starting from the intermediate (S) pipecolic acid 2,6-xylidide (145 g; 0.624 mols) and n-propyl bromide (766 g; 6.24 mols) in tetrahydrofuran (2.5 L) under reflux for approx. 20-24 hours. Inorganic salts are filtered off and the solvent is evaporated to dryness to obtain a dry- solid, consisting of crude Ropivacaine base. The solid is taken up into the minimum amount of diisopropyl ether (200 ml) and filtered under vacuum. The residue is washed on the filter with the same solvent (3 x 150 ml) and dried at 55°C under vacuum to obtain 161 g of Ropivacaine base in a 94% molar yield on the xylidide; HPLC purity = 99.75%; HPLC enantiomeric purity = 99.54%, concentration = 99.5%; loss on drying: 0.3%. EPO
References:
WO2006/133837,2006,A2 Location in patent:Page/Page column 3-4

98717-15-8
234 suppliers
$49.00/25mg

84057-95-4
221 suppliers
$49.00/10mg
33420-15-4
2 suppliers
inquiry

27262-40-4
312 suppliers
$11.00/5g

84057-95-4
221 suppliers
$49.00/10mg
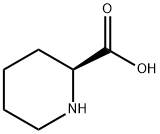
3105-95-1
369 suppliers
$5.00/100mg

84057-95-4
221 suppliers
$49.00/10mg

87-62-7
463 suppliers
$10.00/5G

84057-95-4
221 suppliers
$49.00/10mg