RARECHEM AL BW 0442 synthesis
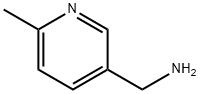
- Product Name:RARECHEM AL BW 0442
- CAS Number:56622-54-9
- Molecular formula:C7H10N2
- Molecular Weight:122.17

6960-22-1

56622-54-9
Example 4: 106 Synthesis of Step 1: Under argon protection, 3.00 g (22.0 mmol) of 6-methylnicotinamide was dissolved in 10 ml of tetrahydrofuran (THF), followed by the slow addition of 34 ml (34 mmol; 1.5 eq.) of 1 M lithium aluminium hydride in THF solution. After 45 min of reaction, 1.22 g (1.5 eq.) of lithium aluminum hydride dissolved in 40 ml of THF was added to the system. The reaction mixture was heated to 60°C under stirring and maintained at this temperature for 24 hours. Upon completion of the reaction, the reaction was carefully quenched sequentially with isopropanol, methanol and water at 0°C under stirring. Subsequently, the solvent was evaporated under reduced pressure. To the residue, 100 ml of 2N NaOH solution and 100 ml of dichloromethane (DCM) were added and centrifuged to separate the resulting white precipitate. The aqueous layer was extracted four times with 60 ml of DCM. All organic layers were combined, dried with anhydrous magnesium sulfate, and concentrated under reduced pressure to remove the solvent to afford 1.57 g (12.9 mmol; 58% yield) of the target product, 3-aminomethyl-6-methylpyridine (amine 2), which was used directly in the subsequent reaction without further purification.
3222-48-8
209 suppliers
$16.00/1 g

56622-54-9
73 suppliers
$52.00/100mg
Yield:56622-54-9 90%
Reaction Conditions:
with ammonia;hydrogen in methanol at 20; for 2 h;
Steps:
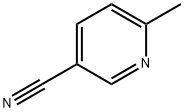
12 Preparation of (6-methylpyridin-3-yl) methanamine
Procedure H: To a mixture of 6-methylnicotinonitrile (300 mg, 2.54 mmol) in MeOH/NH3 (7N, 10mL), was added Raney Ni (50 mg). The reaction mixture was stirred at RT for 3 h under H2. It was then filtered, and the filtrate concentrated in vacuo to give 6- methylpyridin-3-yl) methanamine as a yellow oil (280 mg, 90%). LC-MS (ESI): m/z (M+1)+ = 123.30.
References:
REMEDY PLAN, INC.;CRIMMINS, Gregory, Thomas;DE JESúS DIAZ, Dennise, Alexandra;BHURRUTH-ALCOR, Yushma WO2019/213570, 2019, A1 Location in patent:Paragraph 0331; 0402-0404

6960-22-1
167 suppliers
$39.00/1g

56622-54-9
73 suppliers
$52.00/100mg

474554-80-8
2 suppliers
inquiry

56622-54-9
73 suppliers
$52.00/100mg
28900-10-9
250 suppliers
$9.00/1g

56622-54-9
73 suppliers
$52.00/100mg

4241-27-4
226 suppliers
$6.00/250mg

56622-54-9
73 suppliers
$52.00/100mg