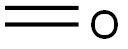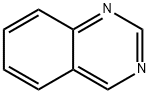
Quinazoline synthesis
- Product Name:Quinazoline
- CAS Number:253-82-7
- Molecular formula:C8H6N2
- Molecular Weight:130.15
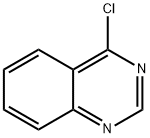
5190-68-1

253-82-7
The general method for the synthesis of quinazoline from 4-chloroquinazoline was as follows: first, the halogenated heterocycle (0.66 mmol) was dissolved in anhydrous THF (13.2 mL) and degassed by drumming argon gas for several minutes. Subsequently, PdCl2 (dppf) (27.0 mg, 0.033 mmol, 5.0 mol%), TMEDA (0.130 g, 1.12 mmol, 1.7 equiv), and NaBH4 (42.4 mg, 1.12 mmol, 1.7 equiv) were added sequentially. The reaction mixture was stirred at room temperature for an appropriate time under argon protection, after which it was post-treated according to standard methods.
![2,3,4,5-TETRAHYDRO-1H-BENZO[E][1,4]DIAZEPINE](/CAS/GIF/1904-65-0.gif)
1904-65-0
23 suppliers
inquiry

253-82-7
156 suppliers
$11.00/1g
Yield:253-82-7 78%
Reaction Conditions:
with [Au11(PPh3)8Cl2]Cl;trimethylamine-N-oxide in toluene at 100; for 20 h;Inert atmosphere;Schlenk technique;
Steps:
Oxidative dehydrogenation of indoline:
General procedure: In a 2-neck RBF, 1 (20 mg, 0.0005 mmol) and TMNO (45 mg, 0.6 mmol) were suspended in toluene (4 mL) and to this solution indoline (60 mg, 0.5 mmol) was added. The reaction mixture was then heated to 100 oC for 20 h and it was filtered and solvent evaporated. The crude product obtained was purified by column chromatography using hexane/ethylacetate (7:1) as eluent to give pure indole (47 mg, 79%). A similar procedure was used for the other indolines and N-heterocycles.
References:
Kumaran, Elumalai;Leong, Weng Kee [Tetrahedron Letters,2018,vol. 59,# 44,p. 3958 - 3960] Location in patent:supporting information

5190-68-1
193 suppliers
$16.00/500mg

253-82-7
156 suppliers
$11.00/1g
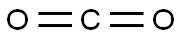
124-38-9
132 suppliers
$175.00/23402

4403-69-4
112 suppliers
$9.00/1g

253-82-7
156 suppliers
$11.00/1g

1904-64-9
2 suppliers
inquiry

271-58-9
116 suppliers
$13.00/250mg

253-82-7
156 suppliers
$11.00/1g