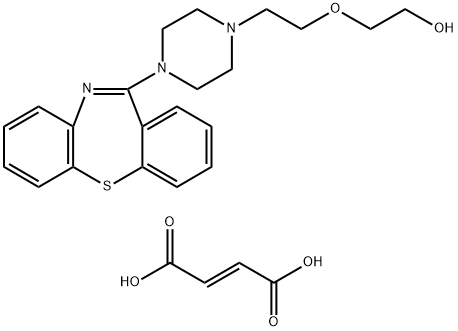
Quetiapine fumarate synthesis
- Product Name:Quetiapine fumarate
- CAS Number:111974-72-2
- Molecular formula:C25H29N3O6S
- Molecular Weight:499.58
![11-(1-PIPERAZINYL)-DIBENZO[B,F][1,4]THIAZEPIN HYDROCHLORIDE](/CAS/GIF/753475-15-9.gif)
753475-15-9

628-89-7

111974-72-2
General procedure for the synthesis of 2-(2-(4-(dibenzo[b,f][1,4]thiazepin-11-yl)piperazin-1-yl)ethoxy)ethan-1-ol hemifumarate from 11-(piperazin-1-yl)dibenzo[b,f][1,4]thiazepin-1-yl)ethoxy)ethan-1-ol: Reagents: 16.5 g (44 mmol) of 11-(piperazin-1-yl)dibenzo[b,f][1,4]thiazepine hydrochloride, 7.2 g (58 mmol) of 2-(2-chloroethoxy)ethanol, 28.5 g (270 mmol) of Na2CO3, 270 mg (0.18 mmol) of NaI, 3 g of TBAB, 82.5 mL of toluene. Steps: 1. add the above reagents to a round-bottomed flask equipped with a magnetic stirrer and a condenser with a calcium chloride drying tube. 2. Place the flask in an oil bath at 105°C and heat under mild reflux conditions. 3. after 24 hours, a Dean-Stark water separator is connected and the azeotropic mixture of water and toluene is distilled. 4. The residue in the flask was filtered and the precipitate (inorganic salt) was washed with a small amount of toluene on a Brinell funnel. 5. Combine the washings with the filtrate and discard the precipitate. 6. 2.6 g (22 mmol) of fumaric acid is added to the filtrate and the mixture is heated to boiling in a heating bath. 7. Remove the flask from the heating bath and continue stirring to precipitate quetiapine hemifumarate crystals. 8. The flask was cooled in an ice bath and the crystalline product was collected by filtration. 9. The resulting solid was recrystallized from 150 ml of ethanol. Yield: 14.0 g, 72% yield.

110-17-8
1100 suppliers
$9.00/5g

111974-69-7
193 suppliers
$25.00/100mg

111974-72-2
733 suppliers
$25.00/100mg
Yield:111974-72-2 99%
Reaction Conditions:
in ethyl acetate;toluene at 20; for 1.08333 h;Product distribution / selectivity;Heating / reflux;
Steps:
2
Example 2; Preparation of quetiapine hemifumarate; A suspension of 1.62 g (0.014 mol) of fumaric acid in 100 ml of ethyl acetate is brought to the boil. At this temperature, 100 ml of a toluene solution containing 10.77 g (0.028 mol) of pure quetiapine base is added, and the reaction mixture is allowed to boil for about 5 minutes.During this period a crystalline product starts to precipitate, which is then cooled to ca. 20 0C and is stirred at this temperature for 60 minutes. After being sucked off and dried, approximately 12.28 g (0.028 mol) of quetiapine hemifumarate is obtained, i.e. 99 % of the theory, having the HPLC purity 99.76 %, and melting point 175.7 0C.
References:
WO2008/3270,2008,A1 Location in patent:Page/Page column 5
![11-(1-PIPERAZINYL)-DIBENZO[B,F][1,4]THIAZEPIN HYDROCHLORIDE](/CAS/GIF/753475-15-9.gif)
753475-15-9
17 suppliers
$197.00/1mL

628-89-7
389 suppliers
$14.00/5g

111974-72-2
733 suppliers
$25.00/100mg

628-89-7
389 suppliers
$14.00/5g

110-17-8
1100 suppliers
$9.00/5g

111974-72-2
733 suppliers
$25.00/100mg

110-17-8
1100 suppliers
$9.00/5g

111974-72-2
733 suppliers
$25.00/100mg
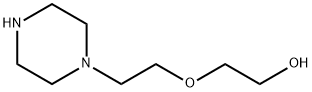
13349-82-1
454 suppliers
$5.00/1g
![Dibenzo[b,f][1,4]thiazepin-11-amine HCl](/CAS/20200331/GIF/1176987-11-3.gif)
1176987-11-3
5 suppliers
inquiry

110-17-8
1100 suppliers
$9.00/5g

111974-72-2
733 suppliers
$25.00/100mg