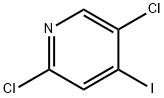
2,5-Dichloro-4-iodopyridine synthesis
- Product Name:2,5-Dichloro-4-iodopyridine
- CAS Number:796851-03-1
- Molecular formula:C5H2Cl2IN
- Molecular Weight:273.89
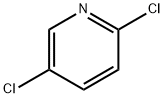
16110-09-1

796851-03-1
a) Dissolve 2,5-dichloropyridine (10 g, 67.57 mmol) in THF (17 mL) under nitrogen protection and slowly add dropwise to a mixture of n-BuLi isohexane solution (33.8 mL, 67.57 mmol) and diisopropylamine (9.63 mL, 67.57 mmol) in THF (68.0 mL) that has been pre-cooled to -78°C. . After the dropwise addition, the reaction mixture was continued to be stirred at -78°C for 30 min. Subsequently, a solution of THF (17.0 mL) with I2 (17.49 g, 68.92 mmol) was added slowly dropwise, and after completion of the dropwise addition, the reaction was kept at -78°C for 1 hour. At the end of the reaction, the reaction was quenched with deionized water (75 mL) and slowly warmed to room temperature. The reaction mixture was extracted with ether (3 x 100 mL), the organic phases were combined, dried with anhydrous MgSO4 and concentrated under reduced pressure. The residue was ground with dichloromethane to give the solid product 2,5-dichloro-4-iodopyridine (9.72 g, 53% yield). The concentrated residue of the filtrate was purified by silica gel column chromatography using a gradient elution with a 50-100% isohexane solution of dichloromethane. The product-containing fraction was collected, concentrated under reduced pressure, and the residue was ground with methanol to give a second batch of 2,5-dichloro-4-iodopyridine (5.74 g, 31% yield).1H NMR (300 MHz, DMSO) δ 7.85 (1H, s), 8.34 (1H, s).

16110-09-1
466 suppliers
$5.00/1g

796851-03-1
130 suppliers
$19.00/250mg
Yield:796851-03-1 53%
Reaction Conditions:
Stage #1:2,5 dichloropyridine with n-butyllithium;diisopropylamine in tetrahydrofuran;2-Methylpentane at -78; for 1.5 h;Inert atmosphere;
Stage #2: with iodine in tetrahydrofuran;2-Methylpentane at -78;Inert atmosphere;
Steps:
3.01.a
a) A solution of 2,5-dichloropyridine (10 g, 67.57 mmol) in THF (17 mL) was added dropwise to a stirred solution of n-BuLi in isohexane (33.8 mL, 67.57 mmol) and diisopropylamine (9.63 mL, 67.57 mmol) in THF (68.0 mL) cooled to -780C, over a period of 1 hour under a nitrogen atmosphere. The resulting mixture was stirred at -780C for 30 minutes and then a solution of I2 (17.49 g, 68.92 mmol) in THF (17.0 mL) was added dropwise. The resulting solution was stirred at -78 0C for 1 hour and then quenched with water (75 mL) and allowed to warm to room temperature. The mixture was extracted with Et2O (3x 100 mL) and the combined organic layers were dried over MgSO4, and then evaporated. The residue was triturated with CH2Cl2 to give a solid which was dried under vacuum to afford 2,5-dichloro-4-iodopyridine (9.72 g, 53% yield). The filtrate was evaporated and the residue purified by chromatography on silica, eluting with a gradient of 50-100% CH2Cl2 in isohexane. Fractions containing product were combined and evaporated and the residue triturated with MeOH to leave a second crop of 2,5-dichloro-4- iodopyridine (5.74 g, 31% yield); 1H NMR spectrum: (300 MHz, DMSO) δ 7.85 (IH, s), 8.34 (IH, s).
References:
ASTRAZENECA AB;ASTRAZENECA UK LIMITED WO2009/153589, 2009, A1 Location in patent:Page/Page column 117