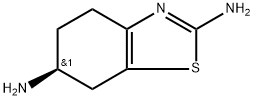
Pramipexole impurity 7 synthesis
- Product Name:Pramipexole impurity 7
- CAS Number:106092-09-5
- Molecular formula:C7H11N3S
- Molecular Weight:169.25
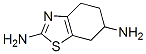
106006-83-1

106092-09-5
General procedure for the synthesis of (S)-2,6-diamino-4,5,6,7-tetrahydrobenzothiazole from 2,6-diamino-4,5,6,7-tetrahydrobenzothiazole: First, 33.8 g of 2,6-diamino-4,5,6,7-tetrahydrobenzothiazole was mixed with 500 mL of water, followed by the addition of 30.0 g of L-(+)-tartaric acid. The reaction mixture was heated to 75°C and stirred for 1 hour. Thereafter, it was cooled to room temperature and stirring was continued for 24 hours. After completion of the reaction, filtration was carried out, the precipitate was washed with water and dried under vacuum at 55°C to 65°C and -0.01 MPa to -0.1 MPa for 8-12 hours to afford 35.1 g of (S)-2,6-diamino-4,5,6,7-tetrahydrobenzothiazolium di-L-(+)-tartrate as crude product with an enantiomeric content of 2.53% (by chiral-HPLC determination). The entire crude product was redissolved in 500 mL of water, heated to 75°C and held for 1 hour, then cooled to room temperature and stirred for 24 hours. It was filtered again, washed with water and dried under the same vacuum conditions for 8-12 hours to give 26.9 g of (S)-2,6-diamino-4,5,6,7-tetrahydrobenzothiazolyl di-L-(+)-tartrate with enantiomeric content reduced to 0.08%. Finally, the entire product was dissolved in a mixture of 200 mL of water and 40 mL of hydrochloric acid and stirred until completely dissolved. Subsequently, 35 drops of aqueous potassium hydroxide solution (mass percent is the ratio of potassium hydroxide to the total mass of the solution) were added to neutralize. The reaction mixture was cooled to room temperature and stirred for 1 h. The reaction mixture was filtered, the precipitate was washed with water and dried under vacuum at 55°C to 65°C and -0.01 MPa to -0.1 MPa for 8-12 h. The final product was 9.43 g of (S)-2,6-diamino-4,5,6,7-tetrahydrobenzothiazole in a yield of 27.9% and enantiomeric content of 0.08%.

873431-80-2
1 suppliers
inquiry

106092-09-5
410 suppliers
$6.00/1g
Yield:106092-09-5 92%
Reaction Conditions:
Stage #1:(S)-4,5,6,7-tetrahydro-benzothiazole-2,6-diamine tartrate trihydrate with water;pyrographite at 80; for 1 h;
Stage #2: with water;potassium hydroxide;sodium hydroxide; pH=12 at 10 - 20; for 0.5 h;Temperature;
Steps:
1A-7A; 1B-3B Example 4A
Add (S) -2,6-diamino-4,5,6,7-tetrahydrobenzothiazole * L-tartrate (0.156mol, 50g), 100ml water to the reaction vessel,Heat to 80 ° C, completely dissolve, add 2 grams of activated carbon and stir for 1 hour.Filtered, and the filtrate was cooled to between 10 ° C and 20 ° C,Add 15% (mass ratio) aqueous solution of sodium hydroxide and potassium hydroxide (NaOH molar amount: KOH molar amount = 1: 1),Until pH = 12.0, a precipitate is precipitated, and stirring is continued for 30 min.Filtration, an appropriate amount of ice water lotion, and drying to obtain 24.3 g of off-white solid, yield 92%,Ignition residue 0.2%, content ≧ 99.5%, single impurity ≦ 0.1%.
References:
Fujian Furui Mingde Pharmaceutical Co., Ltd.;Ren Jianqiang;Zhang Guozu;Zhang Tao;Huang Jianlong CN110669024, 2020, A Location in patent:Paragraph 0023-0042
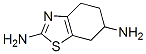
106006-83-1
47 suppliers
inquiry

106092-09-5
410 suppliers
$6.00/1g
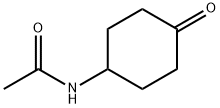
27514-08-5
315 suppliers
$6.00/5g

106092-09-5
410 suppliers
$6.00/1g

106006-80-8
12 suppliers
inquiry

106092-09-5
410 suppliers
$6.00/1g
