
Phenylmethylsulfonyl fluoride synthesis
- Product Name:Phenylmethylsulfonyl fluoride
- CAS Number:329-98-6
- Molecular formula:C7H7FO2S
- Molecular Weight:174.19
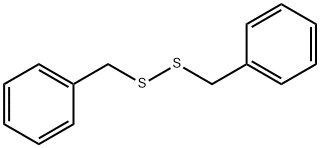
150-60-7

329-98-6
General procedure for the synthesis of phenylmethylsulfonyl fluoride from dibenzyl disulfide: Selectfluor? (2306.2 mg, 6.5 mmol) was added to a stirred mixed solution of dibenzyl disulfide (246.3 mg, 1.0 mmol) in acetonitrile (10.0 mL) and water (1.0 mL). The reaction mixture was heated under reflux conditions for 1.5 h and the progress of the reaction was monitored by thin layer chromatography (TLC). After the dibenzyl disulfide and the corresponding thiosulfonate disappeared on TLC, water (10 mL) was added to the reaction mixture and extracted with ethyl acetate (20 mL × 3). The organic phases were combined, washed with saturated saline, dried over anhydrous magnesium sulfate, and concentrated under reduced pressure. Finally, it was purified by silica gel column chromatography to obtain phenylmethylsulfonyl fluoride (299.0 mg, 86% yield) in colorless crystal form.

1939-99-7
224 suppliers
$11.00/1/ G

329-98-6
399 suppliers
$5.00/250mg
Yield:329-98-6 97%
Reaction Conditions:
with potassium fluoride;triethylamine in dichloromethane;water;
Steps:
18 EXAMPLE 18
EXAMPLE 18 A solution of 95.25 g (0.5 mol) of phenylmethanesulphonic acid chloride in 400 ml of methylene chloride is treated with a solution of 34.8 g (0.6 mol) of potassium fluoride in 80 ml of water. 10 g of triethylamine are added dropwise to the mixture at 25° C. After stirring for 3 hours at room temperature, the organic phase is separated off, dried with sodium sulphate and then freed from the solvent in a rotary evaporator. The residue is distilled in a high vacuum. This gives 84.5 g of phenylmethanesulphonic acid fluoride (boiling point0.5: 80 - 85° C; yield: 97%).
References:
US4060549,1977,A

150-60-7
270 suppliers
$13.00/25g

329-98-6
399 suppliers
$5.00/250mg

36331-57-4
23 suppliers
inquiry

329-98-6
399 suppliers
$5.00/250mg

16601-40-4
0 suppliers
inquiry

329-98-6
399 suppliers
$5.00/250mg