
PHENYL METHACRYLATE synthesis
- Product Name:PHENYL METHACRYLATE
- CAS Number:2177-70-0
- Molecular formula:C10H10O2
- Molecular Weight:162.19

920-46-7

108-95-2

2177-70-0
Phenyl methacrylate was synthesized from methacryloyl chloride and phenol by esterification reaction. The procedure was as follows: phenol (1.0 eq.) was mixed with methacryloyl chloride (1.1 eq.) in anhydrous dichloromethane under dry reaction conditions, triethylamine (1.2 eq.) was added as a base, and the reaction was stirred for 12 hours at room temperature. Upon completion of the reaction, the organic phase was washed with dilute hydrochloric acid to remove unreacted base, followed by washing with saturated sodium bicarbonate solution and water, and finally dried over anhydrous sodium sulfate. The solvent was removed by distillation under reduced pressure to give the crude product. Purification by column chromatography (eluent: petroleum ether/ethyl acetate = 10:1) afforded phenyl methacrylate as a clear oil in 1.45 g yield, 84%. The structure of the product was confirmed by 1H NMR and 13C NMR, and the data were consistent with those reported in the literature.1H NMR (500 MHz, DMSO): δ 7.46-7.41 (m, 2H), 7.30-7.26 (m, 1H), 7.19-7.15 (m, 2H), 6.28 (m, 1H), 5.90 (app p, J=1.55 Hz, 1H ), 2.00 (m, 3H); 13C NMR (125 MHz, DMSO): δ 165.3, 150.6, 135.3, 129.5, 127.7, 125.9, 121.8, 18.1. High-resolution mass spectrometry (HRMS-ESIpos) analysis: calculated value C10H10O2Na [M+Na]+ 185.0572299, measured value 185.057440.
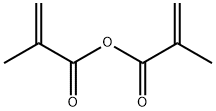
760-93-0
331 suppliers
$28.46/50g

108-95-2
768 suppliers
$14.00/25g

2177-70-0
129 suppliers
$8.00/5g
Yield:2177-70-0 99.1%
Reaction Conditions:
with sulfuric acid at 80; for 8 h;Product distribution / selectivity;
Steps:
19.B
(Step (B)) 696 g (7.4 mol) of phenol and 35 g (0.35 mol) of 98 mass % sulfuric acid were added to the 3 L flask containing the above reaction liquid, and the mixture was heated to 80°C, with air bubbled, and then reacted for 8 hours. The methacrylic anhydride disappeared completely. The yield of phenyl methacrylate based on the methacrylic anhydride (calculated by dividing the number of moles of the phenyl methacrylate at the completion of the reaction by the number of moles of the methacrylic anhydride and multiplying the result by 100) at this time was 99.1 %, and the contained methacrylic acid was 823 g.
References:
EP2308820,2011,A1 Location in patent:Page/Page column 22
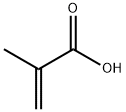
79-41-4
533 suppliers
$14.00/5g
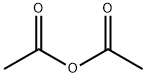
108-24-7
0 suppliers
$14.00/250ML

108-95-2
768 suppliers
$14.00/25g

2177-70-0
129 suppliers
$8.00/5g

122-79-2
254 suppliers
$14.00/1g
