
Octocrylene synthesis
- Product Name:Octocrylene
- CAS Number:6197-30-4
- Molecular formula:C24H27NO2
- Molecular Weight:361.48
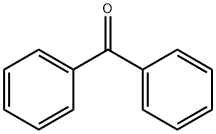
119-61-9

13361-34-7

6197-30-4
To a 1000 mL four-necked round-bottomed flask equipped with a mechanical stirrer, thermometer, and condenser tube, 2-ethylhexyl cyanoacetate (347 g, 1.76 mol), benzophenone (200 g, 1.1 mol), recovered acetic acid (168.3 g with 2% water, 2.75 mol), and ammonium acetate (3 g, 0.4 mol) were added sequentially. Stirring was turned on and the reaction mixture was heated to 85-90 °C. The acetic acid/water mixture was distilled azeotropically under reduced pressure at 400-420 mmHg for 16 h until gas chromatographic analysis showed no further decrease in benzophenone concentration in the reaction mixture. The loss of acetic acid was regulated by making up the recovered acetic acid during the process. Subsequently, the remaining acetic acid was recovered under reduced pressure at 30-60 mmHg. The reaction mixture was analyzed by HPLC and showed a BPCA content of 0.19%. The crude product was cooled, washed twice with 300 g of water and then distilled to give a light yellow viscous liquid (303 g, 76.3% yield) with a purity of 99.48% as analyzed by gas chromatography.
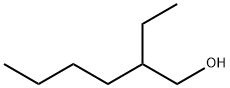
104-76-7
687 suppliers
$5.00/10g

5232-99-5
302 suppliers
$6.00/10g

6197-30-4
446 suppliers
$5.00/250mg
Yield:6197-30-4 99.8%
Reaction Conditions:
in sodium carbonate;
Steps:
1 Preparation of 2-ethylhexyl 2-cyano-3,3-diphenylacrylate STR6
EXAMPLE 1 Preparation of 2-ethylhexyl 2-cyano-3,3-diphenylacrylate STR6 138.5 g (0.5 mole) of ethyl 2-cyano-3,3-diphenylacrylate and 196 g (1.5 mole) of 2-ethylhexanol were caused to react at 130° C. in the presence of 5 g (0.05 mole) of sodium carbonate, the ethanol formed being removed by distillation assisted by a stream of nitrogen (approx. 22 l/h). The transesterification reaction was complete after about one hour, and the solution was filtered from the sodium carbonate while still hot. The purity of the thus obtained 2-ethylhexyl 2-cyano-3,3-diphenylacrylate was already very high at this stage. Purification by film evaporation provided the ester in the form of a light-yellow oil in 97% yield and having a purity of 99.8% as determined by gas-chromatographic analysis. The same results were obtained using, as base, calcium carbonate or magnesium oxide for a reaction period of from 2 to 3 hours.
References:
US5047571,1991,A

119-61-9
819 suppliers
$5.00/10g

13361-34-7
147 suppliers
$15.00/25mL

6197-30-4
446 suppliers
$5.00/250mg

119-61-9
819 suppliers
$5.00/10g

13361-34-7
147 suppliers
$15.00/25mL

731-48-6
0 suppliers
inquiry

6197-30-4
446 suppliers
$5.00/250mg

119-61-9
819 suppliers
$5.00/10g

6197-30-4
446 suppliers
$5.00/250mg