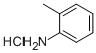
O-TOLUIDINE HYDROCHLORIDE synthesis
- Product Name:O-TOLUIDINE HYDROCHLORIDE
- CAS Number:636-21-5
- Molecular formula:C7H10ClN
- Molecular Weight:143.61
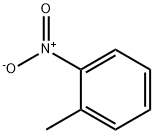
88-72-2

636-21-5
The general procedure for the synthesis of o-biphenylamine hydrochloride from o-nitrotoluene was as follows: the catalyst precursor in the form of 0.04 M CuSO4 and 0.004 M CoCl2 solution (3.0 ml, 3.0 mol/mol CuSO4, 0.3 mol/ml CoCl2) was added to magnetically stirred 25 ml methanol solution of o-nitrotoluene (4 mmol). The reaction was initiated by the addition of an initial portion of 210 mg (5.6 mmol) of NaBH4, and a change in the color of the reaction solution to black (in situ generated catalyst) accompanied by the escape of violent gases was observed. Subsequently, NaBH4 (7 × 35 mg, 7 × 0.9 mmol) was added in portions at 3 min intervals. The reaction was carried out at room temperature and atmospheric pressure, and due to the exothermic nature of the reaction, the temperature of the reaction mixture was usually raised to 30-40 °C, and additional cooling was usually not required for small-scale reactions. For large scale reactions (10 mmol), a reflux condenser needs to be connected. After 25 minutes of reaction, the reaction was quenched by adding 30 ml of water and extracted with 3 x 40 ml of CH2Cl2. The organic layers were combined and dried with MgSO4. After removal of the desiccant, an ether solution (10 ml) of 6 M HCl was added to form a more stable hydrochloride, followed by removal of excess HCl, CH2Cl2, and Et2O under reduced pressure (10 mmHg).The amine products were analyzed by GC-MS, 1H-NMR, and 13C-NMR. The NMR data of the compounds are listed in Table 2.

88-72-2
271 suppliers
$15.00/25g

636-21-5
119 suppliers
$14.00/5g
Yield:636-21-5 545 mg
Reaction Conditions:
Stage #1:1-methyl-2-nitrobenzene with phosgene;sodium tetrahydroborate;copper(II) sulfate in methanol at 20;
Stage #2: with hydrogenchloride in methanol;diethyl ether
Steps:
General Procedure for the Reduction of Aromatic Nitro Compounds
General procedure: The catalyst precursor in form of a 0.04 M CuSO4 and 0.004 M CoCl2 solution (3.0 ml,3.0 mol% CuSO4, 0.3 mol% CoCl2) was added to a magnetically stirred solution ofthe nitro compound (4 mmol) in 25 ml methanol. The reaction was initiated by addingan initial portion of 210 mg (5.6 mmol) NaBH4, resulting in a color change to black(catalyst generated in situ) and vigorous gas evolution. Additional portions of NaBH4(7 × 35 mg, 7 × 0.9 mmol) NaBH4 were added in intervals of 3 min. Although thereaction was carried out at room temperature and under normal atmosphere, the generation of heat due to the exothermic character of the reaction usually heated the reaction mixture to 30-40C and cooling is generally not necessary on a small scale. However,for large scale reactions (10 mmol), a reflux condenser was attached. After 25 min,the reaction mixture was quenched by adding 30 ml of water and extracted with 3 ×40 ml of CH2Cl2. The combined organic layers were dried over MgSO4. After removal of the drying agent, 6 M HCl in ether (10 ml) was added to produce the more stable hydrochloride, which was freed from excess HCl, CH2Cl2 and Et2O under reduced pressure(10 mmHg). An aliquot of the amine was analyzed using GC-MS, 1H-NMR and 13C-NMR. The NMR data for the compounds are shown in Table 2.
References:
Ficker, Mario;Petersen, Johannes F.;Hansen, Jon S.;Christensen, Jorn B. [Organic Preparations and Procedures International,2014,vol. 46,# 2,p. 176 - 182]
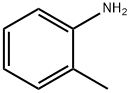
95-53-4
462 suppliers
$10.00/1g

636-21-5
119 suppliers
$14.00/5g
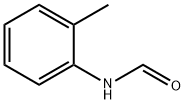
94-69-9
52 suppliers
$27.00/5g

636-21-5
119 suppliers
$14.00/5g