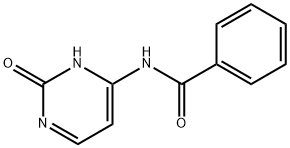
N4-Benzoylcytosine synthesis
- Product Name:N4-Benzoylcytosine
- CAS Number:26661-13-2
- Molecular formula:C11H9N3O2
- Molecular Weight:215.21

71-30-7
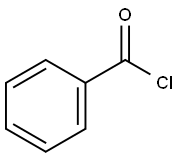
98-88-4

26661-13-2
Under nitrogen protection, 100 L of acetonitrile was added to the reactor as a solvent, followed by 22 kg (196 mol) of 6-aminopyrimidin-2(1H)-one (anhydrous cytosine), 25 g of 4-dimethylaminopyridine (DMAP), and 24 kg of triethylamine as a base. After cooling the reaction mixture to 5-8°C, 34 kg of benzoyl chloride was slowly added dropwise. After the dropwise addition, the reaction mixture was allowed to warm up naturally to room temperature and stirred at 25°C for 1 hour. Subsequently, the reaction mixture was slowly heated to 40-45°C and kept at this temperature for 2 hours. Upon completion of the reaction, it was cooled to room temperature and press-filtered. About 60 L of acetonitrile filtrate was collected for recycling, and the filter cake was washed sequentially with water and ethanol, and dried to give 40 kg of a white solid product, N4-benzoylcytosine, which was analyzed by liquid chromatography to be 99.2% pure.
Yield:26661-13-2 90%
Reaction Conditions:
in pyridine;methanol
Steps:
Monomer Synthesis
Benzoyl chloride (2.3 mL, 20 mmol) was added dropwise over 30 min to a stirred suspension of cytosine (1.1 g, 10 mmol) in dry pyridine, and stirring was continued at room temperature for a further 4 h at room temperature. The reaction was quenched by adding a small amount of methanol. The solid was filtered, washed with EtOH and dried in vacuo to give 1.92 g of the desired product 6 (90%).
References:
NANYANG TECHONOLOGICAL UNIVERSITY US2011/245458, 2011, A1