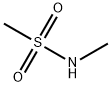
N-Methyl methanesulfonamide synthesis
- Product Name:N-Methyl methanesulfonamide
- CAS Number:1184-85-6
- Molecular formula:C2H7NO2S
- Molecular Weight:109.15

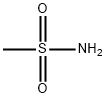
124-63-0

74-89-5

1184-85-6
Methanesulfonyl chloride (100 g, 1.0 eq.) was added slowly and dropwise to a stirred ethanol solution of monomethylamine (8 M in EtOH, 525 mL, 4.8 eq.) at 0 °C. After the dropwise addition, the reaction mixture was warmed to room temperature and stirred continuously for 16 hours. Upon completion of the reaction, the solvent was removed by distillation under reduced pressure. The residue was diluted with dichloromethane (200 mL) and the isolated solid was filtered. The filtrate was concentrated to afford the target product, N-methylsulfonylmethylamine, as an orange oil (82 g, yield not indicated). The product was characterized by 1H NMR (CDCl3, 400 MHz): δ 4.819 (broad single peak, 1H), 2.929 (single peak, 3H), 2.789 (single peak, 3H).
Yield:1184-85-6 82%
Reaction Conditions:
in ethanol at 0 - 20; for 16 h;
Steps:
A.1 Step 1: N-methylmethanesulfonamide
To a stirred solution of methylamine in EtOH (8M in EtOH, 525 mL, 4.8 eq) at 0°C, methane sulfonyl chloride (100 g, 1.0 eq) was added drop wise and stirred at rt for 16 h. After completion, the solvent was distilled off under vacuum and the residue was diluted with DCM (200 mL). The solid that separated out was filtered off and filtrate was concentrated to obtain product N-methylmethanesulfonamide as orange coloured oil (82 g, %). 1H NMR (CDC13, 400 MHz): δ 4.819 (brs, 1H), 2.929 (s, 3H), 2.789 (s, 3H).
References:
ASANA BIOSCIENCES, LLC;VENKATESAN, Aranapakam M.;SMITH, Roger A.;THOMPSON, Scott K.;LAPING, Nicholas;KULKARNI, Bheemashankar;HALLUR, Gurulingappa;VISWANADHAN, Vellarkad N.;PENDYALA, Muralidhar;KETHIRI, Raghava Reddy;TYAGI, Rajiv;SIVANANDHAN, Dhanalakshmi;BAKTHAVATCHALAM, Rajagopal WO2015/38417, 2015, A1 Location in patent:Page/Page column 82

124-63-0
0 suppliers
$10.00/5g

1184-85-6
220 suppliers
$6.00/5g