
N-Methyl-L-prolinol synthesis
- Product Name:N-Methyl-L-prolinol
- CAS Number:34381-71-0
- Molecular formula:C6H13NO
- Molecular Weight:115.17

147-85-3

34381-71-0
(2) 5.0 g (0.0434 mol) of L-proline was dissolved in 92 mL of 97% formic acid and the reaction temperature was maintained at 5°C-10°C. 30 mL of acetic anhydride was added slowly and the reaction mixture was stirred continuously for 2 hours. After completion of the reaction, the reaction was quenched by adding 35 mL of ice water at room temperature. The solvent was removed by evaporation to afford (S)-(-)-N-formylproline ([α]D2?: -105°, c = 2.885, methanol), the product was a clarified, viscous, light yellow oil, which could be used for the next reaction without further purification. Under nitrogen protection, 20 mL of tetrahydrofuran solution of (S)-(-)-N-formylproline was slowly added to 125 mL of tetrahydrofuran suspension of 8.23 g (0.217 mol) of lithium aluminium hydride, and the rate of addition was controlled to maintain mild reflux. After addition, the reaction was continued at reflux for 48 hours. At the end of the reaction, it was cooled to room temperature and the reaction was quenched by careful addition of 8.3 mL of water, 8.3 mL of 15% aqueous sodium hydroxide solution and 25 mL of water in that order. The off-white mixture obtained was filtered and the filtrate was dried over magnesium sulfate and concentrated to an oil. Purification by bulb-to-bulb distillation (oven temperature 40°C-55°C, 0.15 Torr) afforded 2.823 g of N-methyl-L-prolinol in 57% yield (as L-proline).

147-85-3
1064 suppliers
$6.00/25g

34381-71-0
243 suppliers
$16.80/5G
Yield:34381-71-0 57%
Reaction Conditions:
with aq. sodium hydroxide;acetic anhydride in tetrahydrofuran;formic acid;water
Steps:
1.2 A.
(2) To a solution of 5.0 g (0.0434 mol) of L-proline in 92 ml of 97% formic acid maintained at 5°-10° was slowly added 30 ml of acetic anhydride. The solution was stirred for 2 hr. at room temperature followed by the addition of 35 ml of ice-cold water. Evaporation of the mixture afforded (S)-(-)-N-formylproline ([α]D2: =-105°, c=2.885, methanol), a clear, viscous, pale-yellow oil utilized directly in the next step. The (S)-(-)-N-formylproline was reduced by adding a solution of it in 20 ml of tetrahydrofuran to a slurry of 8.23 g (0.217 mol) of lithium aluminum hydride in 125 ml of tetrahydrofuran under a nitrogen atmosphere. The addition was regulated such that a gentle reflux was maintained. Following addition, the mixture was refluxed for 48 hr. After cooling, the mixture was carefully treated with 8.3 ml of water, followed by 8.3 ml of 15% aq. sodium hydroxide, and finally 25 ml of water. The grey-white mixture was filtered, and the filtrate dried with magnesium sulfate and evaporated to an oil. Bulb to bulb distillation [oven temp 40°-55° (0.15 torr)] afforded 2.823 g of V (57% yield based on L-proline).
References:
Philip Morris, Incorporated US4321387, 1982, A
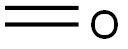
50-00-0
892 suppliers
$10.00/25g
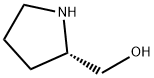
23356-96-9
468 suppliers
$11.19/5G

34381-71-0
243 suppliers
$16.80/5G
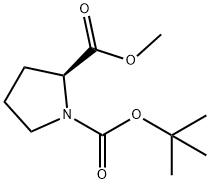
59936-29-7
232 suppliers
$6.00/5g

34381-71-0
243 suppliers
$16.80/5G
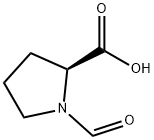
13200-83-4
25 suppliers
$91.09/1g

34381-71-0
243 suppliers
$16.80/5G

1148-11-4
464 suppliers
$6.00/10g

34381-71-0
243 suppliers
$16.80/5G