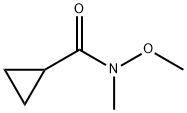
N-METHOXY-N-METHYLCYCLOPROPANECARBOXAMIDE synthesis
- Product Name:N-METHOXY-N-METHYLCYCLOPROPANECARBOXAMIDE
- CAS Number:147356-78-3
- Molecular formula:C6H11NO2
- Molecular Weight:129.16

6638-79-5

4023-34-1

147356-78-3
To a solution of dichloromethane (60 mL) containing N,O-dimethylhydroxylamine hydrochloride (10.0 g, 95.69 mmol, 1.0 eq.) and triethylamine (19.36 g, 191.6 mmol, 2.0 eq.), the reaction mixture was stirred at 0 °C for 30 min. Subsequently, cyclopropanecarbonyl chloride (Compound 96.1) was slowly added at the same temperature. The reaction mixture was transferred to room temperature and continued stirring for 15 hours. After completion of the reaction, the organic layer was washed sequentially with water, brine, saturated sodium bicarbonate solution and 1.0 N hydrochloric acid, dried over anhydrous sodium sulfate and concentrated under reduced pressure to give the crude product. Finally, the crude product was purified by column chromatography to afford N-methoxy-N-methylcyclopropane carboxamide (Compound 96.2, 9.0 g, 72.9% yield).

6638-79-5
596 suppliers
$6.00/25g

1759-53-1
483 suppliers
$6.00/10g

147356-78-3
88 suppliers
$25.00/1g
Yield:-
Reaction Conditions:
with triethylamine;1,1'-carbonyldiimidazole in dichloromethane at 25;
Steps:
B.1
Step l Into a 10-L 4-necked round-bottom flask were placed a solution of cyclopropanecarboxylic acid (500 g, 5.81 mol) in dichloromethane (5000 mL), methoxy(methyl)amine hydrochloride (620 g, 6.36 mol), TEA (1761 g, 17.40 mol), and CDI (1 130 g, 6.97 mol). The resulting solution was stirred overnight at room temperature. The mixture was then diluted with 5000 mL of water and extracted with 2x2000 mL of dichloromethane. The combined organic layers were washed with 2000 mL of brine, dried over anhydrous sodium sulfate, and concentrated under vacuum to afford B2.
References:
MERCK SHARP & DOHME CORP.;DAI, Xing;CUMMING, Jared, N.;LIU, Hong;SCOTT, Jack, D. WO2016/85780, 2016, A1 Location in patent:Page/Page column 48

6638-79-5
596 suppliers
$6.00/25g

4023-34-1
367 suppliers
$12.00/5g

147356-78-3
88 suppliers
$25.00/1g

108817-35-2
17 suppliers
inquiry

6638-79-5
596 suppliers
$6.00/25g

147356-78-3
88 suppliers
$25.00/1g
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

147356-78-3
88 suppliers
$25.00/1g