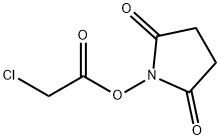
N-(Chloroacetoxy)succiniMide synthesis
- Product Name:N-(Chloroacetoxy)succiniMide
- CAS Number:27243-15-8
- Molecular formula:C6H6ClNO4
- Molecular Weight:191.57

6066-82-6

79-04-9

27243-15-8
GENERAL STEPS: Triethylamine (861.6 μL, 6.18 mmol) was added to a solution of N-hydroxysuccinimide (640.3 mg, 5.56 mmol) in chloroform (8.5 mL) and stirred at 0 °C. Subsequently, α-chloroacetyl chloride was added slowly dropwise over 5 min and stirring was continued at 0 °C for 20 min. Upon completion of the reaction, the reaction mixture was washed with ice-cold water (15 mL) and brine (15 mL) and subsequently concentrated under reduced pressure to a volume of 1.7 mL. The concentrate was dried over anhydrous sodium sulfate and filtered. Ethyl acetate (170 μL) and hexane (1.2 mL) were added to the filtrate and the mixture was cooled to 0 °C and stirred for 2 h to precipitate a white solid. The solid was collected by filtration and washed sequentially with ice-cold hexane/ethyl acetate (4:1, 10 mL), hexane/ethyl acetate (9:1, 10 mL) and hexane (10 mL, twice). Finally, the resulting white solid was dried under vacuum at room temperature to afford 2,5-dioxopyrrolidin-1-yl 2-chloroacetate (563.9 mg, 53% yield). The product was characterized by 400 MHz NMR (CDCl3) with chemical shifts of δ 4.37 (s, 2H), 2.87 (s, 4H).

6066-82-6
797 suppliers
$5.00/10g

79-04-9
382 suppliers
$12.00/5g

27243-15-8
75 suppliers
$41.00/100mg
Yield: 72.9%
Reaction Conditions:
with sodium hydrogencarbonate in dichloromethane at 0 - 20; for 2 h;
References:
Golosov, Andrei A.;Flyer, Alec N.;Amin, Jakal;Babu, Charles;Gampe, Christian;Li, Jingzhou;Liu, Eugene;Nakajima, Katsumasa;Nettleton, David;Patel, Tajesh J.;Reid, Patrick C.;Yang, Lihua;Monovich, Lauren G. [Journal of Medicinal Chemistry,2021,vol. 64,# 5,p. 2622 - 2633]

6066-82-6
797 suppliers
$5.00/10g
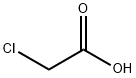
79-11-8
0 suppliers
$24.00/25g

27243-15-8
75 suppliers
$41.00/100mg