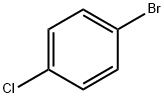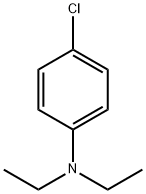
N-(4-chlorophenyl)-N,N-diethylamine synthesis
- Product Name:N-(4-chlorophenyl)-N,N-diethylamine
- CAS Number:2873-89-4
- Molecular formula:C10H14ClN
- Molecular Weight:183.68
Yield:2873-89-4 85%
Reaction Conditions:
with sodium tris(acetoxy)borohydride;acetic acid in dichloromethane at 20;Inert atmosphere;
Steps:
General Experimental Procedure
General procedure: All reactions werecarried out according to the procedure written below unless otherwise noted. The starting anilines (0.1 mmol) in dryCH2Cl2 (2.5 mL) was treated with carboxylic acid (1.3 mmol)and NaBH(OAc)3 (1.6 mmol) at room temperature overnight.Saturated aqueous NaHCO3 was dropped into the reactionmixture, and then the mixture was stirred until the foaming stopped. After extracting with EtOAc, the organic layerwas dried over MgSO4. Removal of solvent from EtOAcextract under reduced pressure by a rotary evaporator gavecrude products that were purified by column chromatography. The obtained products, 4-chloro-N,N-diethylaniline (2),)N-ethylindoline (7),) 1-(4-ethylaminophenyl) ethanone (9),)4-ethylaminobenzophenone (14),) and N-(4-chlorophenyl)-5-methyl-2-pyrrolidone (18)) were confirmed by the previously reported spectroscopic data.
References:
Tamura, Satoru;Sato, Keigo;Kawano, Tomikazu [Chemical and Pharmaceutical Bulletin,2018,vol. 66,# 1,p. 101 - 103]
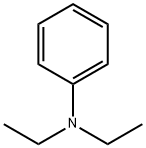
91-66-7
333 suppliers
$9.00/5g

2873-89-4
11 suppliers
inquiry

106-47-8
491 suppliers
$5.00/5G
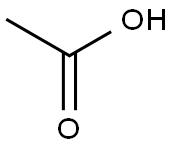
64-19-7
1602 suppliers
$10.00/25ML

2873-89-4
11 suppliers
inquiry
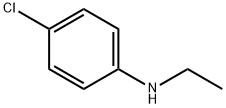
13519-75-0
76 suppliers
$67.00/5g