
MLN-8237 synthesis
- Product Name:MLN-8237
- CAS Number:1028486-01-2
- Molecular formula:C27H20ClFN4O4
- Molecular Weight:518.92

1028486-08-9
![(1E,4E)-8-chloro-4-((diMethylaMino)Methylene)-1-(2-fluoro-6-Methoxyphenyl)-3,4-dihydrobenzo[c]azepin-5-one](/CAS/GIF/869367-33-9.gif)
869367-33-9

1028486-01-2
Synthesis of 4-((9-chloro-7-(2-fluoro-6-methoxyphenyl)-5H-benzo[c]pyrimido[5,4-d]azepine[5,4-d]-2-yl)amino)-2-methoxybenzoic acid from the compound (CAS:1028486-08-9) and 8-chloro-4-[(dimethylamino)methylene]-1-(2-fluoro-6-methoxyphenyl)-3,4-dihydro-5H-2-benzazepin-5-one -2-yl)amino)-2-methoxybenzoic acid in the following general steps: 1. compound 6 (3.81 kg, 15.5 mol), potassium carbonate (4.3 kg, 31.1 mol), compound 9 (5.27 kg, 14.1 mol) and methanol (63 L) were added to the reactor. 2. the suspension was heated to 50 to 55 °C and stirred continuously for at least 24 hours until a conversion of >96.0% was confirmed by HPLC analysis. 3. Methanol (10 L) and water (37 L) were added while keeping the temperature between 50 and 55 °C. 4. The pH of the mixture was adjusted to 3.0 to 4.0 using a 7% w/w HCl solution (prepared from 7.0 kg of concentrated HCl and 24 L of water) while maintaining the temperature between 50 and 55°C. 5. the suspension was cooled to 20 to 25 °C over a period of at least 1 hour and stirring was continued for at least 60 minutes. 6. The resulting suspension was filtered and the filter cake was washed sequentially with water (2 x 26.3 L) at 50 to 55°C and methanol (2 x 10 L) at 20 to 25°C. 7. the wet filter cake was vacuum dried at 45 to 50 °C to give 5.85 kg (80% yield) of the target product formula (II). Product characterization data: 3/4 NMR (300 MHz, DMSO-d6) δ 12.07 (s, 1H), 10.22 (s, 1H), 8.72 (s, 1H), 8.29 (d, J = 8.8 Hz, 1H), 7.95 (s, 1H), 7.80 (dd, J = 2.4, 8.9 Hz, 1H), 7.70 (d, J = 8.8 Hz, 1H) , 7.39 (m, 3H), 7.21 (s, 1H), 6.89 (s, 2H), 3.82 (s, 6H); MS (ESI) m/z 517.2 (M-H+, 45%).

1028486-08-9
19 suppliers
inquiry
![(1E,4E)-8-chloro-4-((diMethylaMino)Methylene)-1-(2-fluoro-6-Methoxyphenyl)-3,4-dihydrobenzo[c]azepin-5-one](/CAS/GIF/869367-33-9.gif)
869367-33-9
11 suppliers
inquiry

1028486-01-2
192 suppliers
$25.00/1mg
Yield:1028486-01-2 80%
Reaction Conditions:
Stage #1:4-{[amino(imino)methyl]amino}-2-methoxybenzoic acid hydrochloride;8-chloro-4-[(dimethylamino)methylene]-1-(2-fluoro-6-methoxyphenyl)-3,4-dihydro-5H-2-benzazepin-5-one with potassium carbonate in methanol at 50 - 55; for 24 h;
Stage #2: with hydrogenchloride in methanol;water; pH=3 at 20 - 55; for 2 h;
Steps:
3.4
4-{f9-chloro-7-(2-fluoro-6-methoxyphenyl)-5H-pyrimido[5,4-rf1[2lbenzazepin-2- ynamino)-2-methoxybenzoic acid (Formula II)[0156] Into a reactor was added 6 (3.81kg, 15.5mol), potassium carbonate (4.3kg, 31.1mol), 9 (5.27kg, 14.1mol) and methanol (63L). The suspension was warmed to 50 to 55 °C and stirred for a minimum of 24h until >96.0% conversion was obtained by HPLC analysis. Methanol (10L) and water (37L) were added while maintaining the temperature between 50 and 55 °C. The pH of the mixture was adjusted to 3.0 to 4.0 using 7% w/ w HC1 (prepared from 7.0kg of concentrated HQ and 24L of water) while maintaining the temperature between 50 and 55 °C. The suspension was cooled to 20 to 25 °C over a minimum of lh and stirred for at least 60min. The resulting suspension was filtered and washed with water (2 x 26.3L) at 50 to 55 °C and methanol (2 x 10L) at 20 to 25 °C. The wet cake was dried at 45 to 50 °C under vacuum to provide 5.85kg (80% yield) of Formula (II). ? NMR (300 MHz, DMSO-d6) δ 12.07 (s, IH), 10.22(s, IH), 8.72 (s, IH), 8.29 (d, J=8.8 Hz, IH), 7.95 (s, IH), 7.80 (dd, J=2.4, 8.9 Hz, IH), 7.70 (d, J=8.8 Hz, IH), 7.39 (m, 3H), 7.21 (s, IH), 6.89 (s, 2H), 3.82 (s, 6H); MS (ESI) m/z MS (ESI) m/z 517.2 (M - H+, 45%).
References:
MILLENNIUM PHARMACEUTICALS, INC.;ARMITAGE, Ian;COOPER, Martin, I.;EDDLESTON, Mark, D.;FAIBER, Neil, C.;MCCUBBIN, Quentin, J.;WATT, Stephen, W. WO2011/103089, 2011, A1 Location in patent:Page/Page column 45

869365-97-9
56 suppliers
$75.00/100mg

1028486-01-2
192 suppliers
$25.00/1mg

28910-83-0
126 suppliers
$11.00/250mg

1028486-01-2
192 suppliers
$25.00/1mg
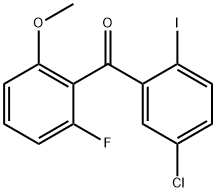
1233025-91-6
30 suppliers
$107.00/25mg

1028486-01-2
192 suppliers
$25.00/1mg
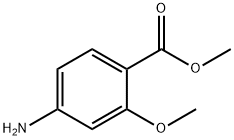
27492-84-8
324 suppliers
$6.00/5g

1028486-01-2
192 suppliers
$25.00/1mg