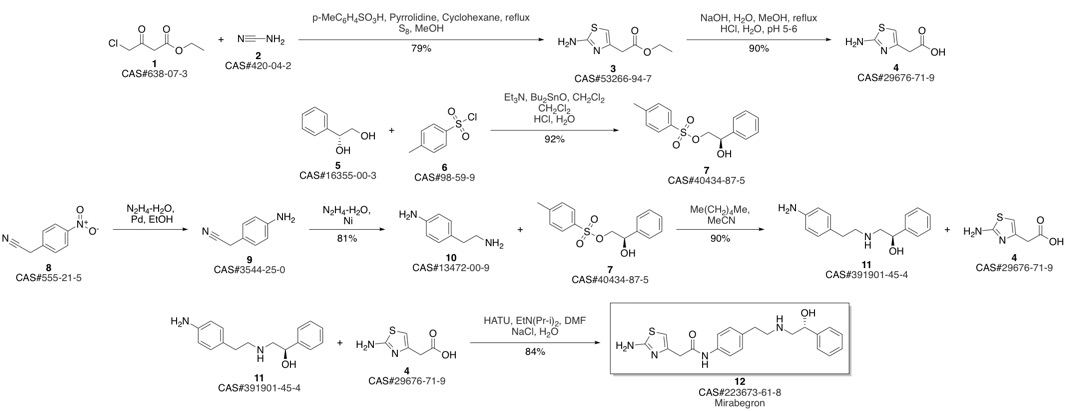
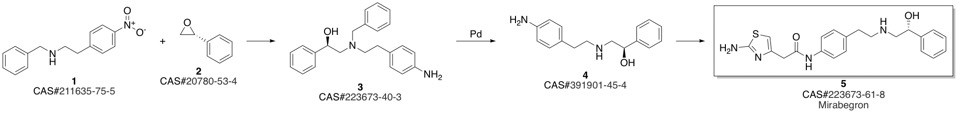
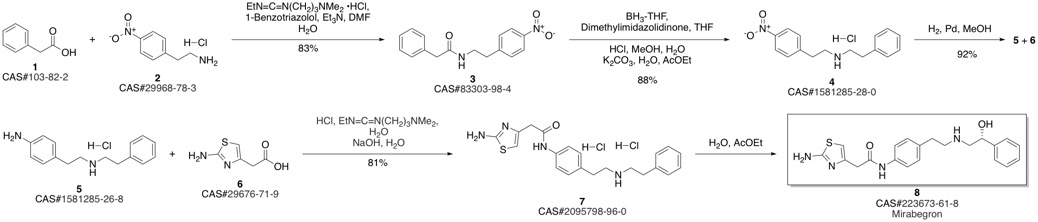
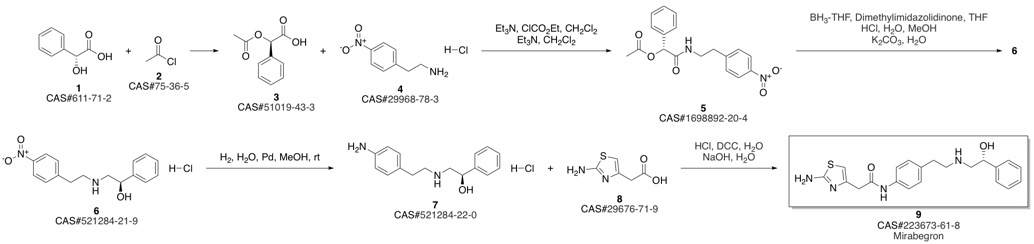
Mirabegron synthesis
- Product Name:Mirabegron
- CAS Number:223673-61-8
- Molecular formula:C21H24N4O2S
- Molecular Weight:396.51

Vedantham, Ravindra; Kandagatla, Bhaskar; Vyala, Sunitha; Raju, V. V. N. K. V. Prasada; Cherukupalli, Praveen; Iqbal, Javed; Dahanukar, Vilas H.; Kagga, Mukkanti; Bandichhor, Rakeshwar; Oruganti, Srinivas. Practical synthesis of Mirabegron. Journal of Chemical and Pharmaceutical Research. Volume 7. Issue 4. Pages 1473-1478. Journal; Online Computer File. (2015).

29676-71-9
454 suppliers
$13.00/5g
![(alphaR)-alpha-[[[2-(4-Aminophenyl)ethyl]amino]methyl]benzenemethanol hydrochloride](/CAS/GIF/521284-22-0.gif)
521284-22-0
195 suppliers
$10.00/250mg

223673-61-8
475 suppliers
$5.00/1mg
Yield:223673-61-8 84.5%
Reaction Conditions:
with hydrogenchloride;1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride in water at 28; for 3 h;Concentration;
Steps:
1d Example idStep d: Preparation of a-form of crystalline (R)-2-(2-aminothiazol-4-yl)-4 ‘-12- F(2-hydroxy- 2-phenylethylaminol -ethyll -acetanilide (Mirabepron)
To a mixed solution of (100 g) (R)-2-[[2-(4-aminophenyl)ethyl]-amino]-l-phenylethanolmonohydrochioride (prepared according to example Ic), 2-aminothiazole-4-yl-acetic acid (55.10 g), concentrated hydrochloric acid (35.61 g) and water (1500 mL); 1-(3-dimethylaminopropyl)-3- ethylcarbodiimide monohydrchloride (EDC.HCI) (72.01 g) was added at 28°C (±2) and the mixture was stirred for 1 hr. l-(3-dimethylaminopropyl)-3-ethylcarbodiimide monohydrochioride (EDC.HCI) (7.2 g) was added into the mixture at 28°C (±2) and the mixture was stirred for 2 hrs. The reaction mass was washed with mixture of ethyl acetate (400 mL) and n-butanol (100 mL). To the reaction mixture, n-butariol (1000 mL) was added followed by addition of aqueous solution of ammonia (20%, 80 mL). The organic layer was separated and successively washed with aqueous ammonia (5%, 1000 mE) and then followed by water. The organic layer was partially concentrated under vacuo and the temperature was raised to 68°C (±2). To this solution, toluene (1400 mE) was added and gradually cooled to room temperature. The solid obtained was filtered, washed with toluene and dried under vacuo at 48°C (±2) to obtain crystals of CL form of Mirabegron having PXRD pattern shown in Fig. 1 and Infrared spectrum (IR) show in Fig 2.Yield: 114.1 g (84.5%); Purity by HPLC: 99.80%.
References:
MEGAFINE PHARMA (P) LTD.;MATHAD, Vijayavitthal Thippannachar;DESHMUKH, Dattatray Gulabrao;VARPE, Sagar Popat;NAVALE, Pravin Mahadu WO2015/44965, 2015, A1 Location in patent:Page/Page column 34; 36

29676-71-9
454 suppliers
$13.00/5g
![BenzeneMethanol, a-[[[2-(4-aMinophenyl)ethyl]aMino]Methyl]-, (aR)-](/CAS/20150408/GIF/391901-45-4.gif)
391901-45-4
102 suppliers
$13.00/1g

223673-61-8
475 suppliers
$5.00/1mg