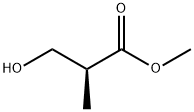
METHYL (S)-(+)-3-HYDROXY-2-METHYLPROPIONATE synthesis
- Product Name:METHYL (S)-(+)-3-HYDROXY-2-METHYLPROPIONATE
- CAS Number:80657-57-4
- Molecular formula:C5H10O3
- Molecular Weight:118.13

15484-46-5

80657-57-4
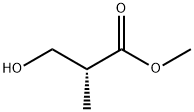
72657-23-9
General procedure for the synthesis of methyl (S)-(+)-3-hydroxy-2-methylpropanoate and (-)-methyl-D-BATA-hydroxyisobutyrate from methyl 2-(hydroxymethyl)acrylate: K.3. Typical method for asymmetric catalytic hydrogenation is as follows: [Rh(COD)L*]BF4 (0.005 mmol, 1 mol%) and substrate (0.5 mmol) were were dissolved in a dry solvent (7.5 mL) and the solution was transferred to a stainless steel autoclave. After sealing the autoclave, it was purged with hydrogen and then pressurized with hydrogen. After stirring the reaction mixture for 16 h at room temperature, the hydrogen was slowly released to atmospheric pressure and the reaction solution was transferred to a round bottom flask. The solvent was removed using a rotary evaporator to obtain the crude product. The crude product was purified by silica gel column chromatography to give the hydrogenated product. The enantiomeric excess value of the product was determined by chiral column HPLC. The results of asymmetric catalytic hydrogenation using rhodium complexes of ligands (1-48) or (1-49) are presented in Tables 9 and 10, respectively. Table 9 demonstrates the results of asymmetric catalytic hydrogenation of rhodium complexes of ligand (1-48) under different conditions including substrate, solvent, pressure, conversion and enantiomeric excess values. Table 10, on the other hand, shows the results of asymmetric catalytic hydrogenation of rhodium complexes of ligand (I'-49c) under the same conditions.

15484-46-5
135 suppliers
$5.00/250mg

80657-57-4
174 suppliers
$15.00/250mg
Yield:80657-57-4 87%
Reaction Conditions:
with diisopropyl{1-[(S)-3,5-dioxa-4-phospha-cyclohepta(2,1-a;3,4-a')dinaphthalen-4-yl]-3-methyl-2-indolyl}phosphine;bis(norbornadiene)rhodium(l)tetrafluoroborate;hydrogen in dichloromethane at -40; under 15001.5 Torr; for 15 h;Reactivity;Inert atmosphere;optical yield given as %ee;enantioselective reaction;Pressure;Temperature;Concentration;
References:
Wassenaar, Jeroen;Kuil, Mark;Reek, Joost N. H. [Advanced Synthesis and Catalysis,2008,vol. 350,# 10,p. 1610 - 1614] Location in patent:supporting information; experimental part

15484-46-5
135 suppliers
$5.00/250mg

80657-57-4
174 suppliers
$15.00/250mg

72657-23-9
212 suppliers
$30.00/1g
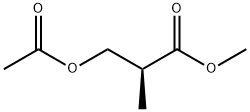
73295-10-0
0 suppliers
inquiry

80657-57-4
174 suppliers
$15.00/250mg

64809-29-6
2 suppliers
inquiry

80657-57-4
174 suppliers
$15.00/250mg

72657-23-9
212 suppliers
$30.00/1g

64809-29-6
2 suppliers
inquiry

80657-57-4
174 suppliers
$15.00/250mg