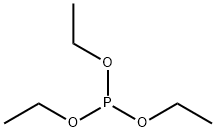
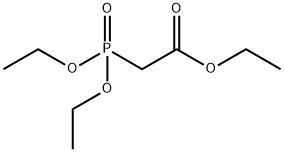
Methyl diethylphosphonoacetate synthesis
- Product Name:Methyl diethylphosphonoacetate
- CAS Number:1067-74-9
- Molecular formula:C7H15O5P
- Molecular Weight:210.16

64-17-5
![Acetic acid, 2-[bis[(trimethylsilyl)oxy]phosphinyl]-, methyl ester](/CAS/20210111/GIF/67605-34-9.gif)
67605-34-9

1067-74-9
TMSBr (90 μL, 0.691 mmol) was added to a solution of anhydrous CH2Cl2 (0.55 mL) of trimethyl phosphonoacetate (2; 50.3 mg, 0.276 mmol) at room temperature. After the reaction mixture was stirred under argon protection at room temperature for 5 h, the solvent was removed by vacuum evaporation to afford methyl 2-[bis[(trimethylmethylsilyl)oxy]phosphono}acetate (4), which was used for the next reaction without further purification. Subsequently, Ph3P (181 mg, 0.691 mmol) and I2 (175 mg, 0.691 mmol) were added to an anhydrous CHCl3 (1.8 mL) solution of 4 under argon protection. The reaction mixture was stirred at room temperature for 15 minutes and then imidazole (188 mg, 2.76 mmol) was added. Stirring was continued at room temperature for 15 minutes, then the reaction mixture was heated to 50°C and held for 30 minutes. Next, 2,2,2-trifluoroethanol (79 μl, 1.10 mmol) was added and the reaction mixture was stirred at 60°C for 5 hours. Upon completion of the reaction, the reaction mixture was filtered and the filtrate was evaporated under vacuum to give the crude product 1. The crude product was purified by column chromatography [silica gel PSQ 60B: n-hexane-EtOAc (2:1)] to give the final product methyl diethylphosphonoacetate (1; 82.3 mg, 94%) as a colorless oil.
Yield:1067-74-9 98%
Reaction Conditions:
at 135; for 10 h;
Steps:
4.11. Preparation of (2E,4E)-5-phenylpenta-2,4-dienoic acid (21)
A mixture of methyl chloroacetate (4.8 g, 44 mmol) and triethylphosphite (7.3 g, 44 mmol) was heated at 135 °C for 10 h. The mixture was allowed to cool to room temperature to afford the desired product, methyl 2-(diethylphosphoryl)acetate, in 98% yield (6.2 g) as a mixture of rotamers.
References:
Aung, Hnin Thanda;Furukawa, Tadashi;Nikai, Toshiaki;Niwa, Masatake;Takaya, Yoshiaki [Bioorganic and Medicinal Chemistry,2011,vol. 19,# 7,p. 2392 - 2396] Location in patent:experimental part
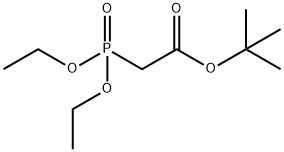
27784-76-5
270 suppliers
$7.00/10g

1067-74-9
219 suppliers
$5.00/5g