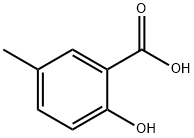
METHYL 5-AMINOSALICYLATE synthesis
- Product Name:METHYL 5-AMINOSALICYLATE
- CAS Number:42753-75-3
- Molecular formula:C8H9NO3
- Molecular Weight:167.16
67-56-1

89-57-6

42753-75-3
In a 5-liter four-necked flask equipped with a reflux condenser, a dropping funnel, a thermometer casing, and a mechanical stirrer, methanol (3500 mL) and 5-aminosalicylic acid (500 g, 3.26 mol) were added and stirring was turned on. The reaction temperature was controlled in the range of 35-40 °C and thionyl chloride (600 mL, 8.16 mol) was added slowly dropwise for about 2 hours. After the dropwise addition, the reaction mixture was heated and refluxed for 15-16 hours, during which the reaction mixture gradually changed to a brown dilute slurry. The progress of the reaction was monitored by thin layer chromatography (TLC) until the residue of 5-aminosalicylic acid was below 1.0% (based on TLC analysis). Subsequently, the following post-treatment was carried out: first, about 2000 mL of methanol was removed by distillation at atmospheric pressure, and then the remaining methanol was azeotropically distilled (3 × 1000 mL) with water at reduced pressure (200-250 mmHg) to obtain a slurry. The slurry was poured into water (3500 mL) and the pH was adjusted first to 5.0 with 25% (w/v) NaOH solution (750 mL) and then to 7.0-7.5 with 20% (w/v) Na2CO3 solution (300 mL).The precipitated solid of methyl 5-amino salicylate was filtered and washed with water (2 x 1000 mL). The filter cake was dried to constant weight at 65 °C under reduced pressure (250 mmHg) to give 490 g of product in 90% yield. Melting point: 93-95°C. Reference: EP 0291159. 1H-NMR (CDCl3) data: δ 3.92 ppm (3H, s, Ar-COOCH3); 6.85 ppm (2H, m, Ar-H); 7.16 ppm (1H, d, J=2.73 Hz, Ar-H). Mass spectral data: m/z 167 (M+), 135, 107, 79.
Yield: 62%
Reaction Conditions:
Stage #1:methanol;5-Methylsalicylic acid with sulfuric acidReflux;
Stage #2: with sodium hydroxide in methanol;dichloromethane;water; pH=7
Steps:
5-Amino-2-hydroxy-benzoic acid methyl ester 19; 5-Methyl salicylic acid (10 g, 65.3 mmol, 1 eq) was dissolved in methanol (80 ml) and conc. H2SO4 (10 ml) was added carefully. The mixture was heated to reflux temperature overnight and was then allowed to cool to ambient temperature and was then poured into a separating funnel and water (100 ml) and DCM (100 ml) were added. The pH was adjusted to 7 with dil. aq. NaOH and the organic layer was poured off. The aqueous layer was then extracted with DCM (2×50 ml) and the combined organic layers were washed with water (2×100 ml), were washed with brine (50 ml), were dried over Na2SO4, filtered and the solvent was removed in vacuo. This gave 9.778 g (62%) of an off white solid. 1H-NMR (DMSO D6) 500 MHz: δ (ppm)=3.85 (3H, s, CH3), 4.78 (2H, br.s, NH2), 6.70 (1H, d, J=8.7 Hz, CCHCHCN), 6.82 (1H, dd, J=2.9 Hz, J=8.7 Hz, CCHCHCN), 7.01 (1H, d, J=2.9 Hz, CCHCN), 9.74 (1H, s, COH). 13CNMR (DMSO D6) 125 MHz: δ (ppm)=52.1 (CH3), 112.1 (C), 112.8 (CH), 117.5 (CH), 123.0 (CH), 141.0 (C), 151.5 (C), 169.6 (CO). IR Spectrum; evaporated film: v(cm-1)=3408, 3328, 3220, 3082, 2958, 1675, 1616, 1485, 1441, 1303, 1231, 1083. MS-ES (positive): 168.06 (M+H+).
References:
Desreumaux, Pierre;Bellinvia, Salvatore;Chavatte, Philippe;Baroni, Sergio US2011/39808, 2011, A1 Location in patent:Page/Page column 50
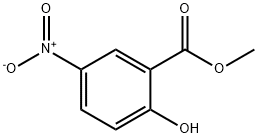
17302-46-4
155 suppliers
$6.00/1g

42753-75-3
99 suppliers
$30.00/1g
67-56-1
782 suppliers
$7.29/5ml-f

89-57-6
803 suppliers
$5.00/50mg

42753-75-3
99 suppliers
$30.00/1g
7664-93-9
0 suppliers
$21.64/1003

89-57-6
803 suppliers
$5.00/50mg

42753-75-3
99 suppliers
$30.00/1g

89-57-6
803 suppliers
$5.00/50mg

42753-75-3
99 suppliers
$30.00/1g