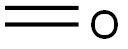METHYL 4-FORMYL-3-HYDROXYBENZOATE synthesis
- Product Name:METHYL 4-FORMYL-3-HYDROXYBENZOATE
- CAS Number:24589-98-8
- Molecular formula:C9H8O4
- Molecular Weight:180.16

67-56-1

619-12-5

24589-98-8
Step 1: Synthesis of methyl 4-formyl-3-hydroxybenzoate (239) 4-Formyl-3-hydroxybenzoic acid (1.2 g, 7.22 mmol) was dissolved in methanol (10 ml). Sulfoxide chloride (1.054 ml, 14.45 mmol) was added slowly and the reaction mixture was stirred at 60 °C for 3 h to ensure complete reaction. After completion of the reaction, the reaction mixture was concentrated under reduced pressure. The crude product was ground with hexane and subsequently filtered to afford methyl 4-formyl-3-hydroxybenzoate (1.3 g, 7.22 mmol, 100% yield) as a white solid. Mass spectrometric analysis (MS/ESI+) showed the molecular ion peak m/z 181.04 [MH]+.
Yield: 80%
Reaction Conditions:
with triethylamine;magnesium chloride in acetonitrile at 0 - 80; for 12 h;Inert atmosphere;Reagent/catalyst;Temperature;
Steps:
1-5 Example 1
Under the protection of inert gas,Compound 1 (methyl 3-hydroxybenzoate) (300g, 1.97mol, 1eq)Add to acetonitrile (4.5L),When the temperature drops to 0 ,At this temperature, 3 equivalents of anhydrous magnesium chloride (564 g, 5.92 mol, 3 eq) were slowly added in portions;When done,Continue to add triethylamine (995g, 9.85mol, 5eq) to the reaction solution in batches at this temperature;When done,Add paraformaldehyde (354g, 11.8mol, 6eq) to the reaction solution in batches at this temperature.After the addition was completed, the temperature was raised to 80 ° C., and the reaction was completed for 12 hours to obtain a reaction solution.The reaction solution was cooled to room temperature, and ethyl acetate (3 L) was added, followed by stirring for 0.5 hours.The reaction solution was poured into a 3M aqueous hydrochloric acid (4L) solution, and the layers were separated.The layers were separated and extracted twice with ethyl acetate (1 L * 2). The organic phases were combined, washed with water, and dried.EA beating and filtering to obtain 283 g of product (yield: 80%, purity 97%)
References:
Shanghai Bide Pharmaceutical Technology Co., Ltd.;Mi Taoran;Tu Qiang CN110407697, 2019, A Location in patent:Paragraph 0039-0054

67-56-1
782 suppliers
$7.29/5ml-f

619-12-5
110 suppliers
$45.00/50mg

24589-98-8
49 suppliers
inquiry

71780-40-0
39 suppliers
$240.00/100mg

24589-98-8
49 suppliers
inquiry
100-97-0
0 suppliers
$14.00/250G
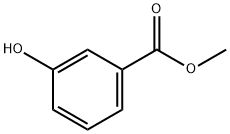
19438-10-9
261 suppliers
$6.00/5g

24589-98-8
49 suppliers
inquiry
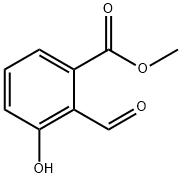
131524-43-1
48 suppliers
inquiry