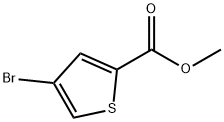
Methyl 4-bromothiophene-2-carboxylate synthesis
- Product Name:Methyl 4-bromothiophene-2-carboxylate
- CAS Number:62224-16-2
- Molecular formula:C6H5BrO2S
- Molecular Weight:221.07

16694-18-1

62224-16-2
b) General procedure for the synthesis of methyl 4-bromothiophene-2-carboxylate: 6.02 g (29.1 mmol) of 4-bromothiophene-2-carboxylic acid (prepared as in the previous procedure) was dissolved in 100 mL of anhydrous methanol under nitrogen protection and cooled to -20 °C. The reaction was completed by the addition of 2.55 mL (34.9 mmol) of thionyl chloride. 2.55 mL (34.9 mmol) of thionyl chloride was slowly added dropwise at a controlled acceleration rate to maintain the reaction temperature below -5 °C (dropwise addition was completed in about 8-10 min). After completion of the dropwise addition, the reaction mixture was stirred at room temperature for 1 hour, followed by refluxing for 8 hours. Upon completion of the reaction, the mixture was cooled and concentrated in vacuum. The resulting 6.7 g of light amber oily product was purified by passing through a 150 g silica gel column, eluting with about 600 mL of dichloromethane (discarding the first 120 mL of the eluate which contained small amounts of impurities). Vacuum concentration gave the title compound, methyl 4-bromothiophene-2-carboxylate, as a colorless oil (6.11 g, 95% yield).1H-NMR (300 MHz, CDCl3) δ 7.69 (d, 1H, J = 1.5 Hz), 7.45 (d, 1H, J = 1.5 Hz), 3.90 (s, 3H).

16694-18-1
213 suppliers
$6.00/1g

62224-16-2
129 suppliers
$13.00/1g
Yield:62224-16-2 98%
Reaction Conditions:
with sulfuric acid in methanol at 65; for 17 h;
Steps:
18.1
A few drops of concentrated sulphuric acid are added to a solution of 5.0 g of the compound obtained during Stage 1 of Preparation 1 in 25 ml of methanol. The reaction medium is stirred at 65° C. for 17 hours and is then concentrated under reduced pressure. The oily residue obtained is dissolved in ethyl acetate (200 ml), washed with water (3×100 ml), dried over sodium sulphate and then concentrated under reduced pressure in order to produce 5.24 g of the desired product. Yield: 98% 1H NMR (CDCl3) δ (ppm): 3.90 (s, 3H), 7.45 (s, 1H), 7.70 (s, 1H)
References:
Dublanchet, Anne-Claude;Compere, Delphine;Cluzeau, Philippe;Blais, Stephane;Denis, Alexis;Ducrot, Pierre;Courte, Karine;Descamps, Sophie US2004/72871, 2004, A1 Location in patent:Page/Page column 29

67-56-1
781 suppliers
$7.29/5ml-f

16694-18-1
213 suppliers
$6.00/1g

62224-16-2
129 suppliers
$13.00/1g

16694-18-1
213 suppliers
$6.00/1g

77-78-1
326 suppliers
$19.69/1ml

62224-16-2
129 suppliers
$13.00/1g

18791-75-8
284 suppliers
$9.00/5g

62224-16-2
129 suppliers
$13.00/1g
6324-10-3
99 suppliers
$6.00/250mg

62224-16-2
129 suppliers
$13.00/1g