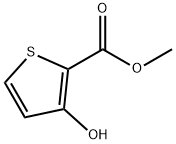
Methyl 3-hydroxythiophene-2-carboxylate synthesis
- Product Name:Methyl 3-hydroxythiophene-2-carboxylate
- CAS Number:5118-06-9
- Molecular formula:C6H6O3S
- Molecular Weight:158.18

80-63-7
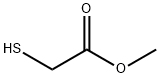
2365-48-2

5118-06-9
Methyl 3-hydroxy-2-thiophenecarboxylate was synthesized according to the method reported by Huddleston and Barker in Synth. Commun. 1979,9,8,731-734. The procedure was as follows: a 2 M solution of sodium methanol was prepared by adding sodium (700 mg; 30 mmol) to 15 mL of anhydrous methanol. Methyl mercaptoacetate (1.9 g; 18 mmol) was then added. The reaction solution was cooled to 0°C and methyl 2-chloroacrylate (2.1 g; 17.4 mmol) was added slowly and dropwise. The reaction mixture was stirred at room temperature overnight. After the reaction was complete, the mixture was cooled to 0°C again and the reaction was quenched with 4 M aqueous hydrochloric acid (~5 mL). Water was added and extracted twice with ethyl acetate. The organic phases were combined, dried over anhydrous sodium sulfate, filtered and concentrated under reduced pressure to give 2.0 g (70% yield) of brown oil, which solidified to a solid product after drying. The product could be used for subsequent reactions without further purification. The product was characterized as follows: LCMS m/z [M-H]-, retention time tR = 4.12 min, purity (UV/MS) 98/20; GCMS m/z 158 (M+), retention time tR = 4.52 min; 1H NMR (CDCl3, 400 MHz) δ 9.56 (br s, 1H, OH), 7.37 (d, 1H, J = 5.2 Hz, thiophene H), 6.74 (d, 1H, J = 5.2 Hz, thiophene H), 3.89 (s, 3H, OMe).
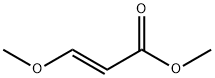
5788-17-0
169 suppliers
$21.00/5mL

2365-48-2
375 suppliers
$15.00/25g

5118-06-9
203 suppliers
$11.00/1g
Yield:5118-06-9 88.2%
Reaction Conditions:
with sodium methylate in methanol under 7500.75 - 15001.5 Torr; for 1 h;
Steps:
1-17 Example 1
The dry clean continuous reactor was heated to 100 °C. Take methyl thioglycolate (91.4g, 0.86mol) and methanol (150g) was placed in a feed flask hit, taking a 30% sodium methoxide in methanol (232.7g, 1.29mol) was placed in flask 2 hits the material, E-3-methoxymethyl acrylate (50.0 g, 0.43 mol) and methanol (150 g) were placed in a shot bottle 3. After the temperature of the continuous coil reactor is stabilized, the material is started, and the feed rate of the shot bottle is set to 10.57 g/min. The feed rate of the shot bottle 2 was set to 0.55 g/min, and the feed rate of the shot bottle 3 was set to 0.47 g/min. The reaction zone retention time is 1 h, and the internal pressure of the continuous coil reactor is controlled at 1.0 to 2.0 MPa. The reaction system was directly passed through a hydrogen chloride/methanol solution, and quenched at a temperature of 0 to 10 °C.After completion of the quenching, the system was concentrated under reduced pressure to give methanol. The residue was purified by distillation under reduced pressure to give 60.1 g of pure white solids, purity 99.5%, yield 88.2 %
References:
Kailaiying Pharmaceutical Group (Tianjin) Co., Ltd.;Hong Hao;Lu Jiangping;Zhang Enxuan;Shen Wei;Guo Pengpeng;Song Di CN109265437, 2019, A Location in patent:Paragraph 0034-0071

80-63-7
123 suppliers
$85.00/5g

2365-48-2
375 suppliers
$15.00/25g

5118-06-9
203 suppliers
$11.00/1g

2365-48-2
375 suppliers
$15.00/25g
623-47-2
351 suppliers
$10.00/1g

5118-06-9
203 suppliers
$11.00/1g

2689-69-2
95 suppliers
$50.70/250mg

5118-06-9
203 suppliers
$11.00/1g
