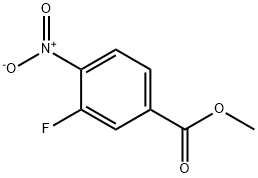
METHYL 3-FLUORO-4-NITROBENZENECARBOXYLATE synthesis
- Product Name:METHYL 3-FLUORO-4-NITROBENZENECARBOXYLATE
- CAS Number:185629-31-6
- Molecular formula:C8H6FNO4
- Molecular Weight:199.14

67-56-1
403-21-4

185629-31-6
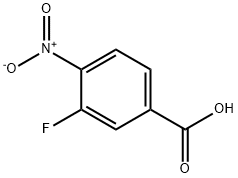
General procedure for the synthesis of methyl 3-fluoro-4-nitrobenzoate from methanol and 3-fluoro-4-nitrobenzoic acid: 3-fluoro-4-nitrobenzoic acid (5.00 g, 27.0 mmol), methanol (40 mL), and an ether solution of 2N HCl were mixed and heated and refluxed for 16 hours. After completion of the reaction, the volatiles were evaporated under reduced pressure. The residue was diluted with ethyl acetate (150 mL), washed sequentially with saturated sodium bicarbonate solution and brine, and dried with anhydrous magnesium sulfate. The solvent was evaporated under reduced pressure to give the title compound methyl 3-fluoro-4-nitrobenzoate (5.18 g, 96% yield) as a light yellow solid.

403-21-4
257 suppliers
$6.00/1g

18107-18-1
210 suppliers
$33.00/5g

185629-31-6
170 suppliers
$5.00/250mg
Yield:185629-31-6 100%
Reaction Conditions:
in methanol;hexane;dichloromethane at 20; for 0.666667 h;Product distribution / selectivity;
Steps:
1x
Compound 236a: 3-Fluoro-4-nitro-benzoic acid methyl ester: A solution of 3-fluoro-4-nitro-benzoic acid (3.0 g, 16.12 mmol, 1.0 eq) in 4:1 DCM/MeOH (50 ml) was stirred at room temperature for 5 minutes and a 2.0M solution of TMS-diazomethane in hexanes (8.1 ml, 16.12 mmol, 1.0 eq) was added drop-wise over 10 minutes, the reaction then stirred at room temperature for 30 minutes. The reaction was quenched with a few drops of acetic acid and the solvent removed in vacuo to give the title compound (3.4 g, 17.09 mmol, 100% corrected). 1H NMR shows the desired product in ca. 90% purity.
References:
DEVELOGEN AKTIENGESELLSCHAFT WO2006/136402, 2006, A1 Location in patent:Page/Page column 119

67-56-1
782 suppliers
$7.29/5ml-f

403-21-4
257 suppliers
$6.00/1g

185629-31-6
170 suppliers
$5.00/250mg

403-21-4
257 suppliers
$6.00/1g

185629-31-6
170 suppliers
$5.00/250mg

67-56-1
782 suppliers
$7.29/5ml-f
157665-51-5
31 suppliers
inquiry

185629-31-6
170 suppliers
$5.00/250mg

403-21-4
257 suppliers
$6.00/1g

75-36-5
591 suppliers
$17.92/100G

185629-31-6
170 suppliers
$5.00/250mg