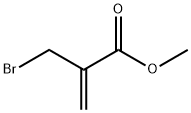
Methyl 2-(bromomethyl)acrylate synthesis
- Product Name:Methyl 2-(bromomethyl)acrylate
- CAS Number:4224-69-5
- Molecular formula:C5H7BrO2
- Molecular Weight:179.01

15484-46-5

4224-69-5
The general procedure for the synthesis of methyl 2-(bromomethyl)acrylate from methyl 2-(hydroxymethyl)acrylate was as follows: 10.2 g of 2-hydroxymethyl methacrylate produced in the previous step was dissolved in 150 mL of acetonitrile, followed by the slow dropwise addition of 4 mL of phosphorus tribromide. After the dropwise addition, the reaction was stirred continuously for 4 hours at room temperature. Upon completion of the reaction, the reaction was quenched by the addition of an appropriate amount of water, followed by removal of the acetonitrile solvent by decompression distillation with a rotary evaporator. To the residue, 100 mL of water was added and extracted twice using ethyl acetate (100 mL each time). The organic phases were combined, dried over anhydrous sodium sulfate and concentrated under reduced pressure to remove the solvent, resulting in 13.5 g of colorless liquid product in 86% yield.

75-18-3
408 suppliers
$18.10/8208330005

15484-46-5
135 suppliers
$5.00/250mg

4224-69-5
161 suppliers
$27.00/1g
Yield:4224-69-5 89%
Reaction Conditions:
with N-Bromosuccinimide;sodium chloride in diethyl ether;dichloromethane
Steps:
Preparation of Methyl 2-bromomethyl-2-propenoate
Preparation of Methyl 2-bromomethyl-2-propenoate To a solution of N-bromosuccinimide (5.17 g, 26.8 mmoles) in dry dichloromethane (40 ml) were added dimethyl sulphide (4 ml) in dichloromethane (50 ml), drop by drop with stirring at 0° C. for 10 min. To the resulting mixture was added methyl 3-hydroxy-2-metylidene-propanoate (3.15 g, 31.50 mmoles) dissolved in dichloromethane (40 ml), leaving it for 24 hours at room temperature. At the end of that time, it was poured into an aqueous solution of sodium chloride and ice. An extraction was performed with diethyl ether (3*100 ml), and the result was washed with water and dried over anhydrous sodium sulphate. Following vacuum concentration, 4.33 g of a yellow oil was obtained (89% yield) of methyl 2-bromomethyl-2-propenoate.
References:
Barrera, Jaime Bermejo;Silva, Margarita Hernandez;De Mon, Melchor Alvarez;Ranieri, Juan Pablo Pivel US2003/199575, 2003, A1

15484-46-5
135 suppliers
$5.00/250mg

4224-69-5
161 suppliers
$27.00/1g
22262-60-8
109 suppliers
$45.00/50mg

4224-69-5
161 suppliers
$27.00/1g

80-62-6
626 suppliers
$18.00/25mL

4224-69-5
161 suppliers
$27.00/1g
![2-Propenoic acid, 2,2'-[oxybis(methylene)]bis-, 1,1'-dimethyl ester](/CAS/20210111/GIF/109669-53-6.gif)
109669-53-6
1 suppliers
inquiry

4224-69-5
161 suppliers
$27.00/1g