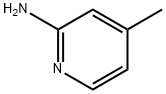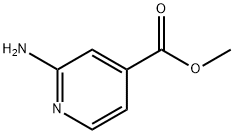
Methyl 2-aminopyridine-4-carboxylate synthesis
- Product Name:Methyl 2-aminopyridine-4-carboxylate
- CAS Number:6937-03-7
- Molecular formula:C7H8N2O2
- Molecular Weight:152.15

67-56-1
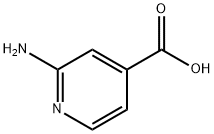
13362-28-2

6937-03-7
The general procedure for the synthesis of methyl 2-aminoisonicotinate from methanol and 2-aminoisonicotinic acid was as follows: 2-aminoisonicotinic acid (207 g, 1.5 mol) was dissolved in methanol (1.8 L) to prepare a solution. The solution was slowly added dropwise to thionyl chloride (238 g, 2 mol) at 50 °C. After the dropwise addition was completed, the reaction temperature was maintained at 50 °C and the reaction mixture continued to be stirred for 5 hours. After completion of the reaction, the solvent was removed by distillation under reduced pressure. To the residue, saturated aqueous sodium carbonate solution was added and the pH was adjusted to 9-10 to make the reaction mixture basic. Subsequently, the mixed solution was extracted with dichloromethane (300 ml each time for 3 times). The organic layers were combined and dried with anhydrous sodium sulfate. Finally, the solvent was removed by concentration to give methyl 2-aminoisonicotinate (189 g, 83% yield) as a yellow solid. The structure of the product was confirmed by 1H-NMR (d6-DMSO, 300 MHz): δ 3.96 (s, 3H); 7.14 (d, 1H); 7.49 (br, 1H); 8.07 (d, 1H); 8.18 (br, 2H, NH).
Yield:6937-03-7 93.9%
Reaction Conditions:
Stage #1:2-Amino-4-methylpyridine with nitric acid in water at 40; for 4 h;
Stage #2:methanol; pH=9 for 2 h;Reflux;Temperature;pH-value;
Steps:
3 Synthesis of 2-aminopyridine-4-carboxylic acid methyl ester
(2) 2-Amino-4-methylpyridine was mixed with water,Heat to 40°C, add Fe-Mn-Mo-TiO catalyst,After stirring, dilute nitric acid is added dropwise.Stir the reaction for 4h,After falling to room temperature,filter,The filtrate was adjusted to pH 9,After adding methanol,After heating and refluxing for 2h,Vacuum distillation to remove methanolMultiple extractions with dichloromethane,Combine the organic phase,Distillation under reduced pressure to remove the dichloromethane,Preparation of 2-aminopyridine-4-carboxylic acid methyl ester.Fe(NO3)3, in step (1)MnCl2,The molar ratio of molybdenum was 4:7:1, the mass ratio of Fe(NO3)3 to nano-TiO2 particles was 1:1, and the molar ratio of NaOH and Na2CO3 was 4:1.In step (2), the mass ratio of 2-amino-4-methylpyridine to Fe-Mn-Mo-TiO catalyst is 11:2.The mass concentration of nitric acid was 30%, the molar ratio of 2-amino-4-methylpyridine to nitric acid was 1:1.5, and the molar ratio of 2-amino-4-methylpyridine to methanol was 1:1.The methyl 2-aminopyridine-4-carboxylate was prepared with a purity of 99.4% and a yield of 93.9%.
References:
Suzhou Aitike Pharmaceutical Chemical Co., Ltd.;Hu Haiwei CN107868040, 2018, A Location in patent:Paragraph 0021-0031; 0032-0038; 0042-0045; 0049-0051

67-56-1
781 suppliers
$7.29/5ml-f

13362-28-2
316 suppliers
$6.00/1g

6937-03-7
214 suppliers
$8.00/1g

58481-11-1
187 suppliers
$6.00/1g

6937-03-7
214 suppliers
$8.00/1g
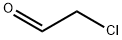
107-20-0
0 suppliers
$18.10/5ml
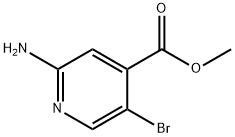
882499-87-8
147 suppliers
$47.00/1g

6937-03-7
214 suppliers
$8.00/1g
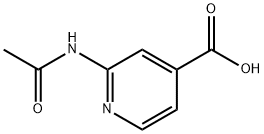
54221-95-3
95 suppliers
$10.00/250mg

6937-03-7
214 suppliers
$8.00/1g