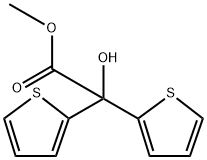
Methyl 2,2-dithienylglycolate synthesis
- Product Name:Methyl 2,2-dithienylglycolate
- CAS Number:26447-85-8
- Molecular formula:C11H10O3S2
- Molecular Weight:254.32
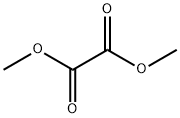
553-90-2

26447-85-8
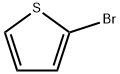
Under nitrogen protection, 2-bromothiophene (9.68 mL, 0.1 mol) was slowly added dropwise to a stirred mixture of magnesium scrapings (2.7 g, 0.11 mol) and ether (100 mL), keeping the reaction temperature at 0 °C. Subsequently, the reaction mixture was warmed up to 35 °C with continuous stirring. Next, a solution of dimethyl oxalate (5.9 g, 0.05 mol) in ether (150 mL) was slowly added dropwise over a period of 3 hours. After the dropwise addition was completed, the reaction mixture was heated to reflux (45 °C) and maintained for 45 minutes. Upon completion of the reaction, the mixture was cooled to room temperature and 1.25 M sulfuric acid (150 mL) was added. After stirring for 1 h at room temperature, the organic layer was separated and washed sequentially with dilute aqueous sodium bicarbonate (100 mL) and water (100 mL). The organic layer was dried over anhydrous sodium sulfate, filtered and concentrated under reduced pressure. The resulting solid residue was recrystallized with carbon tetrachloride (1 g / 3 mL) to afford methyl 2,2-dithienyl ethanoate (7.68 g, 30 mmol, 60% yield).
1003-09-4
488 suppliers
$10.00/25g
26878-13-7
32 suppliers
$220.00/100mg

26447-85-8
285 suppliers
$6.00/1g
Yield:26447-85-8 75%
Reaction Conditions:
Stage #1: 2-bromothiophenewith n-butyllithium in tetrahydrofuran; for 0.333333 h;Inert atmosphere;Cooling;
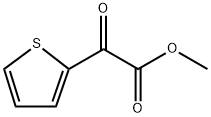
Stage #2: methyl 2-thienylglyoxylate in tetrahydrofuran; for 0.5 h;Inert atmosphere;Cooling;
Steps:
Lithiation of 2-Bromothiophene: preparation of oxo-acetate (4a,c) and glycolates (1a,b).
General procedure: In a flame-dried round flask, organolithium reagent (0.95 mmol) was added dropwise to a solution of 2-bromothiophene 1b (193 mg, 1.0 mmol) in anhydrous THF (15 mL) under N2 at -80 °C. After 20 min, a THF (5.0 mL) solution of oxalate 3 or oxo-acetate 4a-c was added. After the disappearing of the starting thiophene, the reaction was quenched by saturated aqueous NH4Cl and extracted with AcOEt (2×20 mL); the collected organic phases were washed with brine (1×10 mL), dried over Na2SO4 and the solvent was evaporated under vacuum (RV). The resulting crude was purified by FCC - AcOEt/hexane (1:9) - on silica gel. Yield, physical, spectroscopic and analytical data of products 4a,c, 1a,b are as follows.
References:
Foschi, Francesca;Bonandi, Elisa;Mereu, Andrea;Pacchetti, Barbara;Gozzini, Davide;Passarella, Daniele [Arkivoc,2018,vol. 2018,# 7,p. 423 - 430]

1003-09-4
488 suppliers
$10.00/25g

553-90-2
340 suppliers
$10.00/5g

26447-85-8
285 suppliers
$6.00/1g

553-90-2
340 suppliers
$10.00/5g

5713-61-1
49 suppliers
$238.00/50mL

26447-85-8
285 suppliers
$6.00/1g
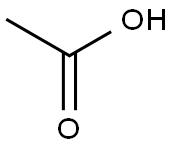
64-19-7
1601 suppliers
$10.00/25ML

26447-85-8
285 suppliers
$6.00/1g
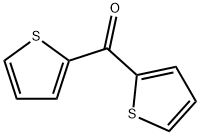
704-38-1
169 suppliers
$12.00/100mg

26447-85-8
285 suppliers
$6.00/1g