
LY2109761 synthesis
- Product Name:LY2109761
- CAS Number:700874-71-1
- Molecular formula:C26H27N5O2
- Molecular Weight:441.52
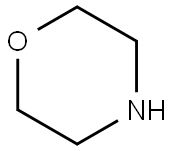
110-91-8
![Ethanol, 2-[[4-[5,6-dihydro-2-(2-pyridinyl)-4H-pyrrolo[1,2-b]pyrazol-3-yl]-7-quinolinyl]oxy]-, 1-methanesulfonate](/CAS/20210305/GIF/700874-75-5.gif)
700874-75-5

700874-71-1
The general procedure for the synthesis of 4-(2-((4-(2-(pyridin-2-yl)-5,6-dihydro-4H-pyrrolo[1,2-b]pyrazol-3-yl)quinolin-7-yl)oxy)ethyl)morpholine from morpholine and the compound (CAS: 700874-75-5) is as follows: 2-[4-(2-pyridin-2-yl-5,6-dihydro-4H- pyrrolo[1,2-b]pyrazol-3-yl)-quinolin-7-yloxy]-ethyl (87 mg, 0.19 mmol) was mixed with morpholine (1 mL) and the reaction was heated at 50 °C for 4 hours. Upon completion of the reaction, excess morpholine was removed by vacuum distillation. Subsequently, the product was separated by extraction using a solvent mixture of isopropanol and chloroform (1:3, v/v). The organic layer was washed with aqueous sodium chloride and dried with anhydrous sodium sulfate. Finally, the title compound (83 mg, 100% yield) was obtained in light yellow solid form by vacuum concentration. Mass spectrometry analysis showed MS ES+ m/e 442.0 ([M+1]+).

110-91-8
707 suppliers
$9.00/1g
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

700874-71-1
141 suppliers
$28.00/1unit
Yield: 100%
Reaction Conditions:
at 50; for 4 h;
Steps:
1.D D. Preparation of 7- (2-MORPHOLIN-4-YL-ETHOXY)-4- (2-PYRIDIN-2-YL-5, 6-dihydro- 4H-PYRROLO [1, 2-B] PYRAZOL-3-YL)-QUINOLINE
Heat METHANESULFONIC acid 2- [4- (2-PYRIDIN-2-YL-5, 6-dihydro-4H-pyrrolo [1, 2- B] PYRAZOL-3-YL) -QUINOLIN-7-YLOXY]-ETHYL ESTER (87 MG, 0.19 MMOL) WITH MORPHOLINE (1 mL) at 50 C for 4 hours. Remove the morpholine in vacuo and then extract the product with isopropyl alcohol : chloroform (1: 3). Wash the organic layer with sodium chloride and dry over sodium sulfate. Concentrate in vacuo to give the desired title product as a slight yellow solid (83 mg, 100%). MS ES+m/e 442.0 (M+1).
References:
ELI LILLY AND COMPANY WO2004/48382, 2004, A1 Location in patent:Page 7