
L-4-Fluorophenylalanine synthesis
- Product Name:L-4-Fluorophenylalanine
- CAS Number:1132-68-9
- Molecular formula:C9H10FNO2
- Molecular Weight:183.18

7761-30-0
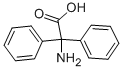
3060-50-2
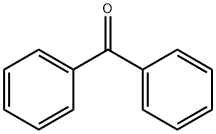
119-61-9

1132-68-9
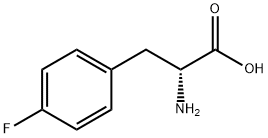
18125-46-7
General procedure for the synthesis of benzophenone, (S)-2-amino-3-(4-fluorophenyl)propanoic acid and (R)-2-amino-3-(4-fluorophenyl)propanoic acid from 4-fluorophenylpyruvic acid and 2,2-diphenylglycine: To a 5 mL reaction vial fitted with a magnetic stirring bar was added 3-cyclohexyl-2-oxypropanoic acid (1j) (0.0510 g, 0.30 mmol ), 2,2-diphenylglycine (2) (0.0681 g, 0.30 mmol), chiral pyridoxamine 6 g (0.0195 g, 0.030 mmol), and MeOH-H2O (8:2, 3.0 mL). The reaction mixture was stirred at 20 °C for 3 days. Subsequently, the reaction mixture was transferred to a 25 mL round-bottomed flask and MeOH was added until all solids were completely dissolved. Next, silica gel (0.50 g) was added. After removal of the solvent by reduced pressure distillation at 20 °C, the resulting residue was separated by silica gel column chromatography (eluent ratio EtOH/ethyl acetate/25-28% ammonia = 100:58:16) to afford compound 3j (0.0401 g, 78% yield, 52% ee) as a white solid. To determine the enantiomeric excess of 3b-k, the compounds were first converted to N-benzoylmethyl esters by treatment with thionyl chloride in methanol followed by reaction with benzoyl chloride and then analyzed by HPLC. For the determination of the enantiomeric excess of 3a, it was analyzed by HPLC after conversion to methyl ester by treatment with CH2N2 in methanol.
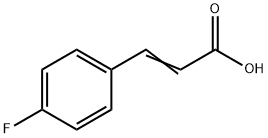
459-32-5
324 suppliers
$6.00/5g

1132-68-9
272 suppliers
$12.50/1G
Yield:-
Reaction Conditions:
with phenylalanine ammonia lyase from Rhodotorula graminis;ammonium carbamate in aq. buffer; pH=9 at 30;Kinetics;Reagent/catalyst;
Steps:
4.6.3 Kinetics in the amination reaction (Assay 2)
General procedure: Solutions of the substrates under investigation were made up in 0.5M ammonium carbamate/1M Tris, pH 9.0 and dilutions were made with the following concentrations: 3, 2, 1, 0.5, 0.2, 0.02, 0.002 and 0mM of cinnamic acid. Scopoletin solution - 288.25mg of scopoletin was dissolved in 50mL dH2O to make a stock solution. This stock solution was then diluted 1/5 to create a working solution that had a fluorescence of approximately 30,000 RFU. The screening was set up in a 96-well plate and each well contained the following: 100μL substrate solution, 10μL HRP (1mg/mL in dH2O), 10μL l-AAO (1 in 10 dilution of stock in dH2O), 40μL scopoletin solution, 40μL purified PAL. Kinetic data was measured on a plate reader at 30°C. Excitation wavelength 360nm; emission wavelength 480nm; 15s interval.
References:
Rowles, Ian;Groenendaal, Bas;Binay, Baris;Malone, Kirk J.;Willies, Simon C.;Turner, Nicholas J. [Tetrahedron,2016,vol. 72,# 46,p. 7343 - 7347]

7761-30-0
25 suppliers
inquiry

3060-50-2
133 suppliers
$20.00/1g

119-61-9
819 suppliers
$5.00/10g

1132-68-9
272 suppliers
$12.50/1G

18125-46-7
215 suppliers
$10.00/250mg

51-65-0
203 suppliers
$12.50/1G

1132-68-9
272 suppliers
$12.50/1G

18125-46-7
215 suppliers
$10.00/250mg

7761-30-0
25 suppliers
inquiry

1132-68-9
272 suppliers
$12.50/1G

207910-84-7
1 suppliers
inquiry

1132-68-9
272 suppliers
$12.50/1G