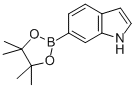
Indole-6-boronic acid pinacol ester synthesis
- Product Name:Indole-6-boronic acid pinacol ester
- CAS Number:642494-36-8
- Molecular formula:C14H18BNO2
- Molecular Weight:243.11

52415-29-9

73183-34-3

642494-36-8
Under nitrogen protection, 6-bromo-1H-indole (25 g, 0.128 mol), pinacol ester of bisboronic acid (48.58 g, 0.191 mol), Pd(dppf)Cl2 (5.2 g, 5 mol%) and KOAc (37.55 g, 0.383 mol) were dissolved in DMF (500 ml). The reaction mixture was stirred at 120°C for several hours. After completion of the reaction, extraction was carried out with ethyl acetate and the organic phase was dried with magnesium sulfate. Purification by column chromatography (eluent: hexane/ethyl acetate=10:1, v/v) afforded 6-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1H-indole (20.15 g, 65% yield).

17422-33-2
335 suppliers
$10.00/1g

73183-34-3
588 suppliers
$6.00/5g

642494-36-8
121 suppliers
$25.00/250 mg
Yield:642494-36-8 78%
Reaction Conditions:
with potassium phosphate tribasic heptahydrate;chloro(2-dicyclohexylphosphino-2′,4′,6′-triisopropyl-1,1′-biphenyl)[2-(2-aminoethyl)phenyl]palladium(II) methyl-t-butyl ether adduct;XPhos in ethanol at 20; for 7 h;
Steps:
18.1 Example 1: Synthesis of 4-tert-butylphenylboronic acid pinacol ester
Method (1): Synthesis from 4-tert-butylchlorobenzene as a raw material: K3P04 · 7H20 (3.0 g, 8.85 mmol) and bis-pinacol borate (749 mg, 2.95 mmol) were sequentially added to the reaction flask.The catalyst-chloro (2-dicyclohexyl phosphino-2 ', 4', 6'-tri - triisopropyl-1,1'-biphenyl) (1,1'-biphenyl -2-amino-2'_ -yl)palladium(II) (12 mg, 0.015 mmol) and ligand 2-dicyclohexylphosphine-2',4',6/-triisopropylbiphenyl (4 mg, 0.008 mmol), followed by EtOH (6 mL) The mixture was stirred, and p-tert-butylchlorobenzene (0.5 mL, 2.95 mmol) was added and the mixture was reacted at room temperature for 0.5 h. After the reaction was completed, the reaction mixture was diluted with ethyl acetate (2 mL) After washing with ethyl acetate (6 mL) in three portions, the filtrate was combined, and the solvent was evaporated to dryness, and the solvent was separated by silica gel (200 to 300 mesh). The eluent was petroleum ether and ethyl acetate. 10~80:1), obtained as a white solid, identified by NMR spectrum as 4-tert-butylphenylboronic acid pinacol ester, yield 98%
References:
Guangzhou Medical University;Ji Hong;Yi Tao;Zhang Chao;Zhang Jianye;Wu Liyang;Cai Jianghong CN109694382, 2019, A Location in patent:Paragraph 0020-0022; 0078-0080

52415-29-9
413 suppliers
$9.00/1g

73183-34-3
588 suppliers
$6.00/5g

642494-36-8
121 suppliers
$25.00/250 mg
399-51-9
356 suppliers
$11.00/5g
25015-63-8
223 suppliers
$22.39/5G

642494-36-8
121 suppliers
$25.00/250 mg

52415-29-9
413 suppliers
$9.00/1g

25015-63-8
223 suppliers
$22.39/5G

120-72-9
654 suppliers
$6.00/25g

642494-36-8
121 suppliers
$25.00/250 mg

52415-29-9
413 suppliers
$9.00/1g

642494-36-8
121 suppliers
$25.00/250 mg