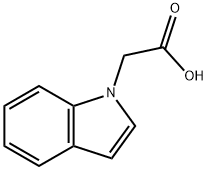
Indol-1-yl-acetic acid synthesis
- Product Name:Indol-1-yl-acetic acid
- CAS Number:24297-59-4
- Molecular formula:C10H9NO2
- Molecular Weight:175.18

61155-69-9

24297-59-4
Step 2: Preparation of 2-(1H-indol-1-yl)acetic acid Ethyl 2-(1H-indol-1-yl)acetate (2.03 g, 10 mmol) was dissolved in ethanol (40 mL), aqueous sodium hydroxide solution (1 N, 50 mL) was added, and the reaction was stirred at room temperature for 1.5 hours. Upon completion of the reaction, the reaction solution was adjusted to slightly acidic with dilute hydrochloric acid and the mixture was subsequently concentrated under reduced pressure. The residue was extracted with ethyl acetate and the organic phase was washed with brine and the solvent was removed under reduced pressure to give pure 2-(1H-indol-1-yl)acetic acid (1.6 g, 87% yield), (LC/MS: m/z = 176.3 [M+H]+).

120-72-9
652 suppliers
$6.00/25g
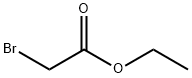
105-36-2
358 suppliers
$5.00/5 g

24297-59-4
120 suppliers
$5.00/250mg
Yield: 92%
Reaction Conditions:
with sodium hydroxide;tetrabutylammomium bromide in water;benzene
Steps:
1 (1H-Indol-1-yl)acetic Acid
EXAMPLE 1 (1H-Indol-1-yl)acetic Acid To a solution of ethyl 2-bromoacetate (25 g, 0.15 mole) and indole (11.7 g, 0.1 mole) in 100 ml of benzene is added a solution of sodium hydroxide (25 g, 0.626 mole) in 50 ml of water. To the obtained mixture is added tetra-n-butylammonium bromide (1.6 g, 0.5 mmole). The mixture is stirred at 25° C. for 16 hours. The aqueous phase is acidified to pH 3. The organic phase is separated, dried and evaporated to give the title compound in 92% yield. NMR (DMSO-D6) ppm (δ) 5.05 (s,2); 6.50 (d, 1), 6.9- 7.8 (m, 5).
References:
Yeda Research and Development Co. Ltd. US4139703, 1979, A

61155-69-9
5 suppliers
inquiry

24297-59-4
120 suppliers
$5.00/250mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

24297-59-4
120 suppliers
$5.00/250mg
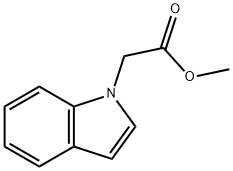
33140-80-6
17 suppliers
inquiry

24297-59-4
120 suppliers
$5.00/250mg

120-72-9
652 suppliers
$6.00/25g

24297-59-4
120 suppliers
$5.00/250mg