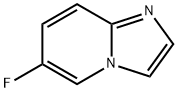
Imidazo[1,2-a]pyridine, 6-fluoro- (9CI) synthesis
- Product Name:Imidazo[1,2-a]pyridine, 6-fluoro- (9CI)
- CAS Number:139022-27-8
- Molecular formula:C7H5FN2
- Molecular Weight:136.13
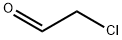
107-20-0
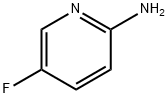
21717-96-4
![Imidazo[1,2-a]pyridine, 6-fluoro- (9CI)](/CAS/GIF/139022-27-8.gif)
139022-27-8
To a solution of 2-amino-5-fluoropyridine (10 g) in ethanol (200 mL) was slowly added 50% aqueous chloroacetaldehyde (56 mL, 4.0 equiv). The reaction mixture was heated under reflux conditions for 2 h and subsequently concentrated under reduced pressure to about 100 mL. The concentrated residue was diluted with ethyl acetate (AcOEt, 100 mL) and washed with saturated aqueous sodium bicarbonate (NaHCO3) (2 x 150 mL). The aqueous phases were combined, saturated with sodium bicarbonate and then back-extracted with ethyl acetate (2 × 100 mL). All organic phases were combined, washed with saturated brine (100 mL), dried over anhydrous sodium sulfate (Na2SO4), and concentrated under reduced pressure to remove the solvent. Purification by fast column chromatography (eluent: dichloromethane solution in 2.5% methanol) afforded 6-fluoroimidazo[1,2-a]pyridine as a milky white solid (8.7 g, 72% yield). Mass spectrum (M + H)+ m/z = 137.

21717-96-4
430 suppliers
$10.00/1g

17157-48-1
31 suppliers
inquiry
![Imidazo[1,2-a]pyridine, 6-fluoro- (9CI)](/CAS/GIF/139022-27-8.gif)
139022-27-8
90 suppliers
$19.00/100mg
Yield:-
Reaction Conditions:
with sodium hydrogencarbonate at 0 - 70;
Steps:
15
Example 15 β-fluoro-imidazofl ,2-α]pydine (26)Synthesis of β-fluoro-imidazofl ,2-α]pydine (26) is shown schematically in Fig. 5. Concentrated HCI (34 ml_, 345 mmol) was added drop-wise to 2-bromo-1 ,1-diethoxyethane 17 (26.37g, 133.8 mmol) at room temperature in a 250-mL three neck round bottomed flask. The mixture was then heated at 550C for 30 minutes. The pale yellow mixture was then cooled to O0C and excess anhydrous Na2SO4 (64g) added and the reaction stirred for 1.0 hr. The reaction mixture now containing crude bromoacetaldehyde 18 was filtered into a three neck 1000 ml. round bottomed flask and the cake washed with ethanol (100 ml_). The flask was then cooled to O0C and 2-amino-5-fluoropyridine (10.Og, 50 mmol) added portion-wise followed by the addition of NaHCO3 (42g, 500 mmol). There was evolution of gas. After the bubbling had ceased, the mixture was refluxed at 7O0C for 1.0 hour. The reaction mixture was then cooled to room temperature, filtered and concentrated under reduced pressure. CH2CI2 was added to the residue at O0C and 40% NaOH (30 ml.) added to pH 10. The mixture was then diluted with water (100 ml.) and the organic phase separated, dried (Na2SO4 ) and concentrated under reduced pressure. Purification on silica gel (CombiFlash, Heptanes/EtOAc elution) afforded 6-fluoro-imidazo[1 ,2-α]pyridine 26 as a brown solid. 5.52g, 85%, yield.1H NMR (300 MHz, CDCI3, 297K) δ 7.06 (m, 1 H), 7.56 (m, 3H), 8.04 (m, 1 H).19F NMR (282.2 MHz, CDCI3, 297K) δ:21.61.ESI MS (MeOH) calcd for C7H5FN2 136.13, found m/z (M++1 ) 137.
References:
ISIS INNOVATION LTD.;EBETINO, Frank, Hallock;MAZUR, Adam;LUNDY, Mark, Walden;RUSSELL, Robert, Graham Goodwin WO2010/33978, 2010, A2 Location in patent:Page/Page column 33
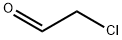
107-20-0
0 suppliers
$18.10/5ml

21717-96-4
430 suppliers
$10.00/1g
![Imidazo[1,2-a]pyridine, 6-fluoro- (9CI)](/CAS/GIF/139022-27-8.gif)
139022-27-8
90 suppliers
$19.00/100mg