
Fostamatinib synthesis
- Product Name:Fostamatinib
- CAS Number:901119-35-5
- Molecular formula:C23H26FN6O9P
- Molecular Weight:580.46

Felfer, Ulfried; Giselbrecht, Karl-Heinz; Wolberg, Michael. Synthesis of N4-(2,2-dimethyl-4-[(dihydrogen phosphonoxy)-3-oxo-5-pyrido[1,4]oxazin-6-yl]-5-fluoro-N2-(3,4,5-trimethoxyphenyl))-2,4-pyrimidinediamine disodium salt. Assignee Rigel Pharmaceuticals, Inc., USA. WO 2011002999. (2011).
![Ditert-butyl [6-[[5-fluoro-2-(3,4,5-trimethoxyanilino)pyrimidin-4-yl]amino]-2,2-dimethyl-3-oxo-pyrido[3,2-b][1,4]oxazin-4-yl]methyl phosphate](/CAS/20180601/GIF/901119-38-8.gif)
901119-38-8
15 suppliers
inquiry

901119-35-5
169 suppliers
$85.00/5mg
Yield:901119-35-5 100%
Reaction Conditions:
with acetic acid in water at 65; for 3 h;
Steps:
1 Synthesis of N4-(2,2-dimethyl-4-[(dihydrogen phosphonoxy)methyl]-3-oxo-5- pyrido[1,4]oxazin-6-yl)-5-fluoro-N2-(3,4,5-trimethoxyphenyl)-2,4-pyrimidinediamine (Compound 4)
N4-(2,2-dimethyl-4-[(di-tert-butyl phosphonoxy)methyl]-3-oxo-5-pyrido[1,4]oxazin-6-yl)- 5-fluoro-N2-(3,4,5-trimethoxyphenyl)-2,4-pyrimidinediamine (Compound 3); (15.0 g, 21.67 mmol) dissolved in AcOH:H20 (225 mL, 4:1) was heated at 650C (oil bath temp). The progress of the reaction was monitored by in process LC/MS. The reaction mixture transformed to faint tan white solid after 1h of heating. At this point most of Compound 3 converted to mono des t-butyl product. After 3h of heating, consumption of SM and complete conversion of intermediate (mono des t- butylated) to product was observed. Reaction mixture was cooled, poured onto ice-water (200 mL), stirred for 20 min and filtered. The clear white filter cake was washed with water (600 ml) and acetone (200 mL) successively, dried for 2h followed by drying under high vacuum over P2O5 in a desiccator. Weight of the solid: 12.70 g; purity: 97% (Compound 3) and 3% (Compound 1) 1H NMR indicated acetic acid presence (1:1) To remove acetic acid, the solid was taken in acetonitrile (300 mL) and concentrated by rotovap vacuum. This process was repeated 2 times with acetonitrile and toluene (3 X 300 mL). The solid obtained was dried under high vacuum at 500C. Finally, the solid was taken in acetone (400 mL), filtered and dried to provide N4-(2,2- dimethyl-4-[(dihydrogen phosphonoxy)methyl]-3-oxo-5-pyrido[1,4]oxazin-6-yl)-5-fluoro-N2- (3,4,5-trimethoxyphenyl)-2,4-pyrimidinediamine (Compound 4). 1H NMR (DMSO-d6): d 9.21 (br s, 2H), 8.16 (d, 1H, J = 2.6 Hz), 7.93 (d, 1H, J = 8.5 Hz), 7.39 (d, 1H, J = 8.5 Hz), 7.05 (s, 2H), 5.79 (d, 1H, J3PH = 6.6 Hz), 3.67 (s, 6H), 3.59 (s, 3H), 1.44 (s, 6H). LCMS: ret. time: 8.52 min.; purity: 95%; MS (m/e): 581 (MH+).31P NMR (DMSO-d6): -2.17.
References:
RIGEL PHARMACEUTICALS, INC. WO2021/30526, 2021, A1 Location in patent:Page/Page column 40-41
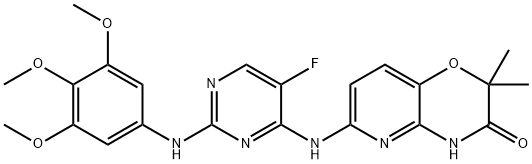
841290-80-0
178 suppliers
$28.00/1unit

901119-35-5
169 suppliers
$85.00/5mg
