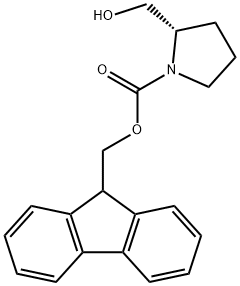
FMOC-PRO-OL synthesis
- Product Name:FMOC-PRO-OL
- CAS Number:148625-77-8
- Molecular formula:C20H21NO3
- Molecular Weight:323.39
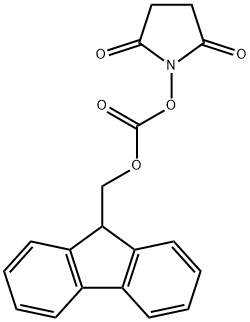
82911-69-1
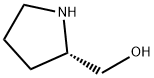
23356-96-9

148625-77-8
(1) Synthesis of Fmoc-L-prolinol (Compound 2): L-prolinol (Compound 1) (0.61 g, 6.0 mmol) was dissolved in 70 mL of deionized water to prepare an aqueous solution of L-prolinol. N-(9-Fluorenylmethoxycarbonyloxy)succinimide (Fmoc-OSu) (2.0 g, 6.0 mmol) was dissolved in 10 mL of tetrahydrofuran (THF). This THF solution was slowly added to the aqueous L-prolinol solution and the reaction was stirred for 1 hour at room temperature. After completion of the reaction, the reaction mixture was separated into liquid and solid phases. These two portions were extracted with ethyl acetate, respectively, and the organic layers were combined. The combined organic layers were dried by adding anhydrous sodium sulfate to the combined organic layers. The desiccant was removed by filtration and the filtrate was concentrated under reduced pressure to obtain the crude product. The crude product was purified by silica gel column chromatography (eluent: hexane/ethyl acetate=1:1, v/v) to afford compound 2 (1.4 g, 74% yield). The nuclear magnetic resonance hydrogen spectrum (1H-NMR) data of the compound were as follows: 1H-NMR (CDCl3): δ 7.77 (2H, d, J = 7.7 Hz, Ar-H), 7.60 (2H, d, J = 7.3 Hz, Ar-H), 7.40 (2H, t, J = 7.5 Hz, Ar-H), 7.31 (2H, t, J = 7.6 Hz Ar-H), 4.40-4.50 (2H, m, COOCH2), 4.22 (1H, t, J = 6.5 Hz, Ar-CH), 3.20-3.80 (5H, m, H-5, H-6), 1.75 (3H, m, H-3, H-4), 1.40 (1H, m, H-3).

82911-69-1
649 suppliers
$7.00/25g

23356-96-9
468 suppliers
$11.19/5G

148625-77-8
94 suppliers
$8.00/250mg

71989-31-6
529 suppliers
$6.00/5g

148625-77-8
94 suppliers
$8.00/250mg

186202-18-6
3 suppliers
inquiry

148625-77-8
94 suppliers
$8.00/250mg
