
FMOC-NLE-OH synthesis
- Product Name:FMOC-NLE-OH
- CAS Number:77284-32-3
- Molecular formula:C21H23NO4
- Molecular Weight:353.41
![4-Hexenoic acid, 2-[[(9H-fluoren-9-ylmethoxy)carbonyl]amino]-, (2S)-](/CAS/20210305/GIF/947154-63-4.gif)
947154-63-4

77284-32-3
V-Fmoc allylglycine (100 mg, 0.296 mmol), degassed dichloromethane (6.0 mL), terminal olefins (1.48 mmol, 5 eq.), and HGII catalyst (9.29 mg, 5 mol%) were added to a Schlenk reaction flask equipped with a magnetic stirring bar in a drying oven under nitrogen atmosphere. After sealing the reaction flask, it was removed from the desiccator and connected to a vacuum system. The reaction vial was placed under a stream of nitrogen and the quick assembly stopper was replaced with a sub-seal with a 26-gauge pinhole to ensure a continuous flow of nitrogen across the top of the reaction system. The reaction mixture was stirred overnight at room temperature, followed by evaporation of all dichloromethane. The residue was washed with hexane (2 x 10 mL) and collected by filtration or centrifugation. The resulting residue was redissolved in methanol (10 mL) and transferred to a Fischer-Porter reaction tube. The reaction tube was filled with hydrogen (60 psi), sealed and stirred overnight at room temperature. Upon completion of the reaction, the mixture was concentrated under vacuum and the residual brown solid was purified by column chromatography to give the pure Fmoc amino acid analog. Alternatively, the residual brown solid is dissolved in a small amount of ether, the insoluble material is removed by filtration, and the filtrate is concentrated under vacuum to give an off-white solid.

327-57-1
326 suppliers
$6.00/1g
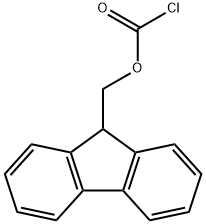
28920-43-6
550 suppliers
$5.00/5g

77284-32-3
255 suppliers
$20.00/1g
Yield:-
Reaction Conditions:
Stage #1:L-Norleucine with sodium carbonate in 1,4-dioxane;waterCooling with ice;
Stage #2:(fluorenylmethoxy)carbonyl chloride in 1,4-dioxane;water at 20; for 5 h;
Steps:
General procedure: The N-FMOC derivatizedracemic and L-amino acids and methyl esters were preparedaccording to conventional methods.22 Racemic or L-α-amino acid (5 mmol) was dissolved in 10% aqueoussodium carbonate solution (12.5 mmol). Dioxane (7.5 mL)was then added and the mixture was stirred in an ice-bath.After that, 9-FMOC chloride (5 mmol) was added slowlyand stirred at room temperature for 5 h. Now, the reactionmixture was poured into water and extracted with ether.The aqueous solution obtained was acidified with c-HCl inan ice-bath. Finally, the resulting N-FMOC α-amino acidwas filtered and dried under vacuum. In order to prepareracemic or L-FMOC α-amino acid methyl ester, the correspondingFMOC α-amino acid (1 mmol) synthesized in theprevious step was dissolved in 5 mL of anhydrous methanolwith N,N0-dicyclohexylcarbodiimide (1.1 mmol). Themixture was stirred at room temperature for 12 h, filteredand dried under vacuum to get N-FMOC α-amino acidmethyl ester.
References:
Islam, Md. Fokhrul;Adhikari, Suraj;Paik, Man-Jeong;Lee, Wonjae [Bulletin of the Korean Chemical Society,2019,vol. 40,# 4,p. 332 - 338]

28920-43-6
550 suppliers
$5.00/5g

77284-32-3
255 suppliers
$20.00/1g

112883-41-7
134 suppliers
$7.00/250mg
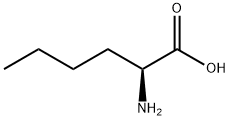
616-06-8
270 suppliers
$12.00/1mg

77284-32-3
255 suppliers
$20.00/1g

112883-41-7
134 suppliers
$7.00/250mg