
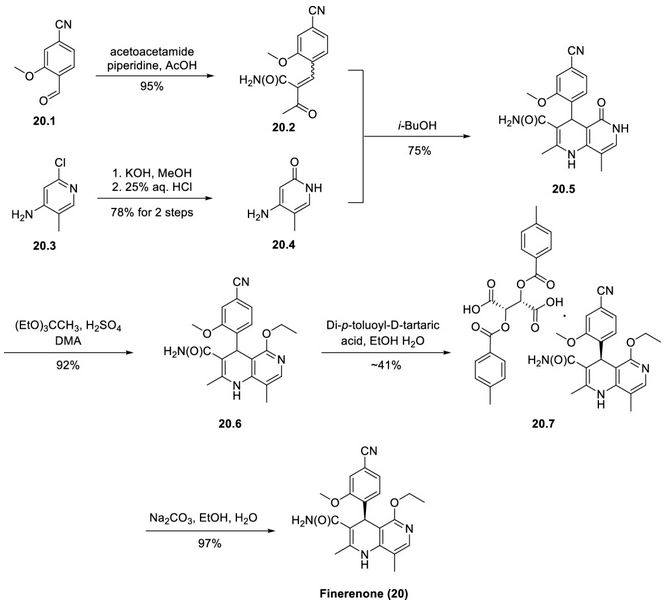
Finerenone synthesis
- Product Name:Finerenone
- CAS Number:1050477-31-0
- Molecular formula:C21H22N4O3
- Molecular Weight:378.42
Addition of 20.2 to a heated isobutanol solution of 20.4 resulted in a condensation-cyclisation reaction to give the nitrogen-containing heterocyclic compound 20.5 in good yield. The conversion of the pyridone to the corresponding ether was achieved by treating the pyridone with 1,1,1-triethoxyethane under acidic conditions. Some of the synthetic methods for fenaridones were carried out by chromatographic separation of the racemate 20.6, while a recent patent reports a classical splitting using tartaric acid derived salts. In one example, 20.6 was reacted with p-toluoyl-d-tartaric acid in a mixture of ethanol and water to give the target enantiomer in 78% enantiomeric excess, which was purified to 99% enantiomeric excess by re-suspension in ethanol and water to ultimately give 20.7 in about 41% yield. Adjusting the pH in water and ethanol with sodium carbonate gave the free base of fenaridone (20) in 97% yield.
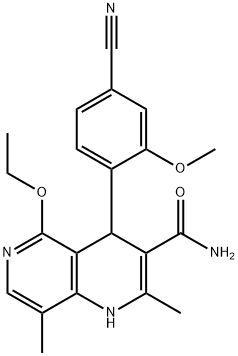
1050477-27-4
73 suppliers
inquiry

29119-58-2
0 suppliers
inquiry
Yield:29119-58-2 80%
Reaction Conditions:
Stage #1: 4-(4-cyano-2-methoxyphenyl)-5-ethoxy-2,8-dimethyl-1,4-dihydro-1,6-naphthyridine-3-carboxamide in 5,5-dimethyl-1,3-cyclohexadiene; for 8 h;Reflux;
Stage #2: with O,O'-dibenzoyl-D-tartaric acid in lithium hydroxide monohydrate at 30; for 4 h;Reflux;Solvent;
Steps:
1.6; 2.7; 3.7 (6) Synthesis of compound 6:
The solvent xylene was added to the reaction flask, 70 g (0.185 mol) of compound 5 was added, 30 g of catalyst was added, and the mixture was refluxed and stirred for 8 h. Target configuration measured by HPLC: non-target configuration = 84:16, ending transconfiguration. Filtration, the mother liquor was spin-dried to obtain the crude product, 150g of ethanol was added, 50g of water was added, after stirring and dissolving, 58.4g (0.155mol) D-dibenzoyltartaric acid was added, heated to reflux for 3h, cooled to 30°C, stirred for 1h and filtered to obtain The crude product was added to water, 10% sodium phosphate solution was added to adjust pH=7.5, stirred for 0.5 h to precipitate, filtered to obtain the crude product, and recrystallized with 200 g of ethanol to obtain 56 g of feneridone bulk drug with a yield of 80%.
References:
CN114605410,2022,A Location in patent:Paragraph 0046; 0064-0066; 0067; 0076-0077; 0087; 0091-0092
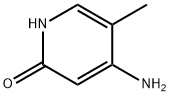
95306-64-2
284 suppliers
$31.00/250mg

29119-58-2
0 suppliers
inquiry