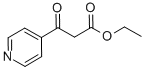
ETHYL ISONICOTINOYLACETATE synthesis
- Product Name:ETHYL ISONICOTINOYLACETATE
- CAS Number:26377-17-3
- Molecular formula:C10H11NO3
- Molecular Weight:193.2

1570-45-2
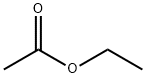
141-78-6

26377-17-3

141-97-9
GENERAL METHOD: Lithium bis(trimethylsilyl)amide (LiHMDS, 30 mmol) was rapidly added to a mixed solution of tetrahydrofuran (THF, 20 mL) and ethyl acetate (70 mmol) of ethyl isonicotinate (10 mmol) at -40°C, maintained at this temperature and stirred for 20 minutes. Upon completion of the reaction, the reaction mixture was quenched with acetic acid (50 mmol) and subsequently alkalized with 10% sodium bicarbonate (NaHCO3) solution. The reaction mixture was extracted with ethyl acetate (2 x 100 mL) and the organic layers were combined and washed sequentially with water and saturated saline. The organic layer was dried with anhydrous sodium sulfate (Na2SO4). Specific purification steps and characterization data are included for each compound.

1570-45-2
386 suppliers
$7.00/10g

141-78-6
665 suppliers
$20.50/250ml

26377-17-3
165 suppliers
$6.00/250mg

141-97-9
821 suppliers
$5.00/25g
Yield: 87%
Reaction Conditions:
with lithium hexamethyldisilazane in tetrahydrofuran at -40; for 0.333333 h;Inert atmosphere;Claisen condensation;
Steps:
General procedure to synthesis β-keto esters from the corresponding esters
General procedure: To the solution of ester (10 mmol) in THF (20 mL) and ethyl acetate (70 mmol), LiHMDS (30 mmol) was added very quickly at -40 °C, and stirred at this temperature for 20 min. After completion of the reaction, reaction mixture was quenched with acetic acid (50 mmol) and then basified using 10% NaHCO3 solution, extracted with ethyl acetate (2×100 mL), the combined organic layer was washed with water and brine solution and dried over Na2SO4. The specific purification procedure for each compound has been included along with their characterization data.
References:
Venkat Ragavan;Vijayakumar;Rajesh;Palakshi Reddy;Karthikeyan;Suchetha Kumari [Bioorganic and Medicinal Chemistry Letters,2012,vol. 22,# 12,p. 4193 - 4197] Location in patent:supporting information; experimental part

1570-45-2
386 suppliers
$7.00/10g

141-78-6
665 suppliers
$20.50/250ml

26377-17-3
165 suppliers
$6.00/250mg
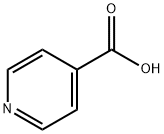
55-22-1
641 suppliers
$5.00/5g

26377-17-3
165 suppliers
$6.00/250mg

55-22-1
641 suppliers
$5.00/5g
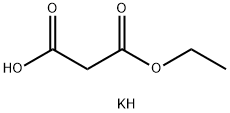
6148-64-7
408 suppliers
$5.00/10g

26377-17-3
165 suppliers
$6.00/250mg
14254-57-0
19 suppliers
$1681.00/10g

6148-64-7
408 suppliers
$5.00/10g

26377-17-3
165 suppliers
$6.00/250mg